Utilization of Waste Leather Powders for Highly Effective Removal of Dyes from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CL Powder
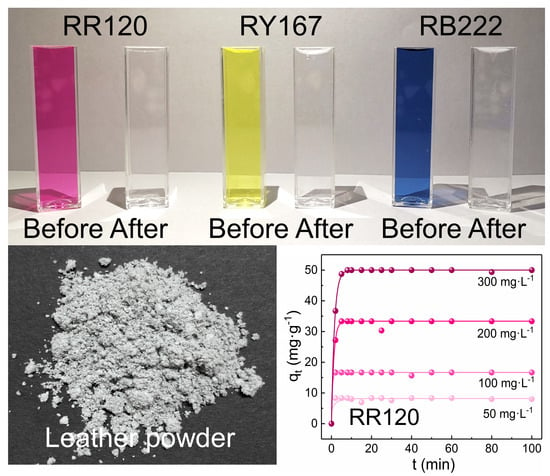
2.3. Adsorption Study
2.4. Characterization
3. Results and Discussion
3.1. Characterization of CL and CL Powder
3.2. Adsorption Isotherms
3.3. Adsorption Kinetics
3.4. Comparison of CL with Other Powders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, N.; Revenga, C.; Echeverria, J. Managing water for people and nature. Science 2001, 292, 1071–1072. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Desai, A.V.; Let, S.; Ghosh, S.K. Advanced Porous Materials for Sensing, Capture and Detoxification of Organic Pollutants toward Water Remediation. ACS Sustain. Chem. Eng. 2019, 7, 7456–7478. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L. A technical review of emerging technologies for energy and water efficiency and pollution reduction in the textile industry. J. Clean. Prod. 2015, 95, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Gopakumar, D.A.; Arumughan, V.; Pottathara, Y.B.; Sisanth, K.S.; Pasquini, D.; Bracic, M.; Seantier, B.; Nzihou, A.; Thomas, S.; et al. Robust Superhydrophobic Cellulose Nanofiber Aerogel for Multifunctional Environmental Applications. Polymers 2019, 11, 495. [Google Scholar] [CrossRef]
- Xia, L.; Wang, A.; Zhang, C.; Liu, Y.; Guo, H.; Ding, C.; Wang, Y.; Xu, W. Environmentally friendly dyeing of cotton in an ethanol-water mixture with excellent exhaustion. Green Chem. 2018, 20, 4473–4483. [Google Scholar] [CrossRef]
- Yokwana, K.; Kuvarega, A.T.; Mhlanga, S.D.; Nxumalo, E.N. Mechanistic aspects for the removal of Congo red dye from aqueous media through adsorption over N-doped graphene oxide nanoadsorbents prepared from graphite flakes and powders. Phys. Chem. Earth 2018, 107, 58–70. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Z.; Wang, Z.; He, Y. Preparation of hierarchically porous carbon from cellulose as highly efficient adsorbent for the removal of organic dyes from aqueous solutions. Ecotoxicol. Environ. Saf. 2019, 168, 298–303. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; Kim, D.S. Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using Banana Peel Powder as an adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607. [Google Scholar] [CrossRef]
- Guo, W.; Xia, T.; Pei, M.; Du, Y.; Wang, L. Bentonite Modified by Allylamine Polymer for Adsorption of Amido Black 10B. Polymers 2019, 11, 502. [Google Scholar] [CrossRef]
- Xue, J.; Song, F.; Yin, X.-W.; Zhang, Z.-L.; Liu, Y.; Wang, X.-L.; Wang, Y.-Z. Cellulose Nanocrystal-Templated Synthesis of Mesoporous TiO2 with Dominantly Exposed (001) Facets for Efficient Catalysis. ACS Sustain. Chem. Eng. 2017, 5, 3721–3725. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Li, L.; Li, W.; Yang, C. Dual Functional N-Doped TiO2-Carbon Composite Fibers for Efficient Removal of Water Pollutants. ACS Sustain. Chem. Eng. 2018, 6, 12893–12905. [Google Scholar] [CrossRef]
- Allègre, C.; Moulin, P.; Maisseu, M.; Charbit, F. Treatment and reuse of reactive dyeing effluents. J. Membr. Sci. 2006, 269, 15–34. [Google Scholar] [CrossRef]
- Capar, G.; Yetis, U.; Yilmaz, L. Membrane based strategies for the pre-treatment of acid dye bath wastewaters. J. Hazard Mater. 2006, 135, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Jayaramudu, T.; Sadiku, E.R. Removal of dye by carboxymethyl cellulose, acrylamide and graphene oxide via a free radical polymerization process. Carbohydr. Polym. 2017, 164, 186–194. [Google Scholar] [CrossRef]
- Daneshvar, N.; Khataee, A.R.; Rasoulifard, M.H.; Pourhassan, M. Biodegradation of dye solution containing Malachite Green: Optimization of effective parameters using Taguchi method. J. Hazard Mater. 2007, 143, 214–219. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, Z.; Ma, H.; Yang, H.; Xu, W. Effective removal of dyes from aqueous solution using ultrafine silk fibroin powder. Adv. Powder Technol. 2014, 25, 574–581. [Google Scholar] [CrossRef]
- Kolomaznik, K.; Adamek, M.; Andel, I.; Uhlirova, M. Leather waste—Potential threat to human health, and a new technology of its treatment. J. Hazard Mater. 2008, 160, 514–520. [Google Scholar] [CrossRef]
- Przepiorkowska, A.; Chronska, K.; Zaborski, M. Chrome-tanned leather shavings as a filler of butadiene-acrylonitrile rubber. J. Hazard Mater. 2007, 141, 252–257. [Google Scholar] [CrossRef]
- Ogino, H.; Otsubo, T.; Ishikawa, H. Screening, purification, and characterization of a leather-degrading protease. Biochem. Eng. J. 2008, 38, 234–240. [Google Scholar] [CrossRef]
- Ravichandran, K.; Natchimuthu, N. Vulcanization characteristics and mechanical properties of natural rubber-scrap rubber compositions filled with leather particles. Polym. Int. 2005, 54, 553–559. [Google Scholar] [CrossRef]
- El-Sabbagh, S.H.; Mohamed, O.A. Recycling of chrome-tanned leather waste in acrylonitrile butadiene rubber. J. Appl. Polym. Sci. 2011, 121, 979–988. [Google Scholar] [CrossRef]
- Santos, R.J.; Agostini, D.L.S.; Cabrera, F.C.; Budemberg, E.R.; Job, A.E. Recycling leather waste: Preparing and studying on the microstructure, mechanical, and rheological properties of leather waste/rubber composite. Polym. Compos. 2014, 36, 2275–2281. [Google Scholar] [CrossRef]
- Ambrósio, J.D.; Lucas, A.A.; Otaguro, H.; Costa, L.C. Preparation and characterization of poly (vinyl butyral)-leather fiber composites. Polym. Compos. 2011, 32, 776–785. [Google Scholar] [CrossRef]
- Mohamed, O.A.; El Sayed, N.H.; Abdelhakim, A.A. Preparation and characterization of polyamide-leather wastes polymer composites. J. Appl. Polym. Sci. 2010, 118, 446–451. [Google Scholar] [CrossRef]
- Swarnalatha, S.; Ganesh Kumar, A.; Tandaiah, S.; Sekaran, G. Efficient and safe disposal of chrome shavings discharged from leather industry using thermal combustion. J. Chem. Technol. Biot. 2009, 84, 751–760. [Google Scholar] [CrossRef]
- Yılmaz, O.; Cem Kantarli, I.; Yuksel, M.; Saglam, M.; Yanik, J. Conversion of leather wastes to useful products. Resour. Conserv. Recycl. 2007, 49, 436–448. [Google Scholar] [CrossRef]
- Deng, D.; Liao, X.; Shi, B. Synthesis of porous carbon fibers from collagen fiber. ChemSusChem 2008, 1, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.G.; Castro, I.A.; Bastos, A.R.; Souza, G.A.; de Carvalho, J.G.; Oliveira, L.C. Recycling of solid waste rich in organic nitrogen from leather industry: Mineral nutrition of rice plants. J. Hazard Mater. 2011, 186, 1064–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazău, R.I.; Păcurariu, C.; Becherescu, D.; Ianoş, R. Ceramic pigments with chromium content from leather wastes. J. Eur. Ceram. Soc. 2007, 27, 1899–1903. [Google Scholar] [CrossRef]
- Kamranifar, M.; Khodadadi, M.; Samiei, V.; Dehdashti, B.; Noori Sepehr, M.; Rafati, L.; Nasseh, N. Comparison the removal of reactive red 195 dye using powder and ash of barberry stem as a low cost adsorbent from aqueous solutions: Isotherm and kinetic study. J. Mol. Liq. 2018, 255, 572–577. [Google Scholar] [CrossRef]
- Chen, F.; Xiong, L.; Cai, M.; Xu, W.; Liu, X. Adsorption of direct fast scarlet 4BS dye from aqueous solution onto natural superfine down particle. Fiber Polym. 2015, 16, 73–78. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Dong, B.; Zhou, Y.; Hu, H.; Xiao, X.; Liu, K.; Zhang, C.; Yuan, T.; Liang, Z.; et al. Preparation of superfine down particles derived from down fiber wastes and their application as an efficient adsorbent toward acid brilliant scarlet 3R. Text. Res. J. 2015, 86, 1050–1062. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Liu, K.; Xiao, X.; Zhang, C.; Cheng, F.; Xia, L.; Zhou, Y.; Dong, B.; Wan, L.; et al. The adsorption performance of Vat Scarlet R on natural superfine needle down particles. Color. Technol. 2016, 132, 28–34. [Google Scholar] [CrossRef]
- Xu, W.; Cui, W.; Li, W.; Guo, W. Development and characterizations of super-fine wool powder. Powder Technol. 2004, 140, 136–140. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, W.; Ma, M.; Zhou, H. Effect of plasma treatment of silk fibroin powder on the properties of silk fibroin powder/polyurethane blend film. Poly. Eng. Sci. 2010, 50, 1705–1712. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Cui, W.; Peng, X.; Li, W.; Liu, X. Characterization of superfine down powder. J. Appl. Polym. Sci. 2009, 111, 2204–2209. [Google Scholar] [CrossRef]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- Papic, S. Removal of some reactive dyes from synthetic wastewater by combined Al (III) coagulation/carbon adsorption process. Dyes Pigments 2004, 62, 291–298. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Chen, S.; Xue, Y.; Du, Z.; Wang, P. Rapid and effective preparation of a HPEI modified biosorbent based on cellulose fiber with a microwave irradiation method for enhanced arsenic removal in water. J. Mater. Chem. A 2016, 4, 15851–15860. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.-H.; Jiang, J.-J.; Wang, H.-P.; Wei, Z.-W.; Zhu, X.; Pan, M.; Su, C.-Y. A stable metal cluster-metalloporphyrin MOF with high capacity for cationic dye removal. J. Mater. Chem. A 2018, 6, 17698–17705. [Google Scholar] [CrossRef]
- Mamane, H.; Kohn, C.; Adin, A. Characterizing Shape of Effluent Particles by Image Analysis. Sep. Sci. Technol. 2008, 43, 1737–1753. [Google Scholar] [CrossRef]
- Walton, W.H. Feret’s Statistical Diameter as a Measure of Particle Size. Nature 1948, 162, 329–330. [Google Scholar] [CrossRef]
- Mazzoli, A.; Favoni, O. Particle size, size distribution and morphological evaluation of airborne dust particles of diverse woods by Scanning Electron Microscopy and image processing program. Powder Technol. 2012, 225, 65–71. [Google Scholar] [CrossRef]
- Chen, J.; Gao, J.; Wu, J.; Zhang, M.; Cai, M.; Xu, H.; Jiang, J.; Tian, Z.; Wang, H. Revealing the carbohydrate pattern on a cell surface by super-resolution imaging. Nanoscale 2015, 7, 3373–3380. [Google Scholar] [CrossRef] [PubMed]
- Kaye, B.H. Some aspects of the efficiency of statistical methods of particle size analysis. Powder Technol. 1968, 2, 97–110. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Zhong, W.; Lu, Y.; Yu, T. pH sensitivity and ion sensitivity of hydrogels based on complex-forming chitosan/silk fibroin interpenetrating polymer network. J. Appl. Polym. Sci. 1997, 65, 2257–2262. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Baggett, J.; Godin, B.; Liu, X.; Kharlampieva, E. Hydrogen-Bonded Multilayers of Silk Fibroin: From Coatings to Cell-Mimicking Shaped Microcontainers. ACS Macro Lett. 2012, 1, 384–387. [Google Scholar] [CrossRef]
- Li, C.; Xue, F.; Ding, E. Fabrication and characterisation of ultrafine leather powder: A functional reinforcement containing SiO2 particles. Micro Nano Lett. 2014, 9, 308–311. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, C.; Xu, W.; Zhu, K.; Wang, A.; Tian, Y.; Wang, Y.; Xu, W. Protective Bleaching of Camel Hair in a Neutral Ethanol(-)Water System. Polymers 2018, 10, 730. [Google Scholar] [CrossRef]
- Xu, W.; Guo, W.; Li, W. Thermal analysis of ultrafine wool powder. J. Appl. Polym. Sci. 2003, 87, 2372–2376. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Xu, W. Thermal and structural characterization of superfine down powder. J. Therm. Anal. Calorim. 2012, 111, 259–266. [Google Scholar] [CrossRef]
- Değermenci, G.D.; Değermenci, N.; Ayvaoğlu, V.; Durmaz, E.; Çakır, D.; Akan, E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Clean. Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Perez-Ameneiro, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Physicochemical study of a bio-based adsorbent made from grape marc. Ecol. Eng. 2015, 84, 190–193. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, P.; Ghosh, R.; Paul, S.; Mondal, S.; Panja, A.; Nandi, A.K. Folic Acid-Polyaniline Hybrid Hydrogel for Adsorption/Reduction of Chromium (VI) and Selective Adsorption of Anionic Dye from Water. ACS Sustain. Chem. Eng. 2017, 5, 9325–9337. [Google Scholar] [CrossRef]
- Chen, H.; Wageh, S.; Al-Ghamdi, A.A.; Wang, H.; Yu, J.; Jiang, C. Hierarchical C/NiO-ZnO nanocomposite fibers with enhanced adsorption capacity for Congo red. J. Coll. Interface Sci. 2019, 537, 736–745. [Google Scholar] [CrossRef]
- Qi, G.; Hai, C.; Shen, Y.; Zeng, J.; Li, X.; Ren, X.; Sun, Y.; Dong, S.; Zhou, Y. Synthesis of mono-dispersed mesoporous Mn2O3 powders with micro-nanostructure for removing Congo red dye from aqueous solution. Adv. Powder Technol. 2019, 30, 930–939. [Google Scholar] [CrossRef]
- Kim, H.R.; Jang, J.W.; Park, J.W. Carboxymethyl chitosan-modified magnetic-cored dendrimer as an amphoteric adsorbent. J. Hazard Mater. 2016, 317, 608–616. [Google Scholar] [CrossRef]
- Chen, J.; Sheng, Y.; Song, Y.; Chang, M.; Zhang, X.; Cui, L.; Meng, D.; Zhu, H.; Shi, Z.; Zou, H. Multimorphology Mesoporous Silica Nanoparticles for Dye Adsorption and Multicolor Luminescence Applications. ACS Sustain. Chem. Eng. 2018, 6, 3533–3545. [Google Scholar] [CrossRef]
- Perez-Ameneiro, M.; Bustos, G.; Vecino, X.; Barbosa-Pereira, L.; Cruz, J.M.; Moldes, A.B. Heterogenous Lignocellulosic Composites as Bio-Based Adsorbents for Wastewater Dye Removal: A Kinetic Comparison. Water Air Soil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- Perez-Ameneiro, M.; Vecino, X.; Barbosa-Pereira, L.; Cruz, J.M.; Moldes, A.B. Removal of pigments from aqueous solution by a calcium alginate-grape marc biopolymer: A kinetic study. Carbohydr. Polym. 2014, 101, 954–960. [Google Scholar] [CrossRef]
- Perez-Ameneiro, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Wastewater treatment enhancement by applying a lipopeptide biosurfactant to a lignocellulosic biocomposite. Carbohydr. Polym. 2015, 131, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Razmi, F.A.; Ngadi, N.; Wong, S.; Inuwa, I.M.; Opotu, L.A. Kinetics, thermodynamics, isotherm and regeneration analysis of chitosan modified pandan adsorbent. J. Clean. Prod. 2019, 231, 98–109. [Google Scholar] [CrossRef]
- Kumar, K.V.; Kumaran, A. Removal of methylene blue by mango seed kernel powder. Biochem. Eng. J. 2005, 27, 83–93. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Sharma, A. Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder. Dyes Pigments 2005, 65, 51–59. [Google Scholar] [CrossRef]









| Dyes | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| Qm (mg·g−1) | Kl (L·mg−1) | R2 | Kf (mg·g−1) (L·mg−1)1/n | 1/n | R2 | |
| RR120 | 167.0 | 0.0229 | 0.9813 | 7.5268 | 0.5548 | 0.9231 |
| RY167 | 178.9 | 0.0174 | 0.9953 | 6.4474 | 0.5946 | 0.9863 |
| RB222 | 129.5 | 0.0300 | 0.9824 | 8.0707 | 0.4877 | 0.9622 |
| C0 (mg·L−1) | Dyes | Qe,exp (mg·g−1) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | Qe,cal (mg·g−1) | R2 | k2 (g·mg−1·min−1) | Qe,cal (mg·g−1) | R2 | |||
| 50 | RR120 | 7.9861 | 0.0187 | 1.0179 | 0.3029 | 0.4502 | 8.0626 | 0.9984 |
| RY167 | 8.5526 | 0.0336 | 1.1972 | 0.5204 | 0.0706 | 8.6118 | 0.9920 | |
| RB222 | 7.1078 | 0.0034 | 1.6949 | 0.1497 | 0.1909 | 6.9818 | 0.9934 | |
| 100 | RR120 | 16.667 | 0.0467 | 1.7518 | 0.5170 | 0.4081 | 16.667 | 0.9999 |
| RY167 | 15.278 | 0.0151 | 1.5394 | 0.1910 | 0.0602 | 15.258 | 0.9956 | |
| RB222 | 16.071 | 0.0068 | 1.4082 | 0.2124 | 0.0831 | 15.654 | 0.9893 | |
| 200 | RR120 | 33.378 | 0.0411 | 1.1530 | 0.5745 | 0.3771 | 33.378 | 0.9999 |
| RY167 | 30.503 | 0.0104 | 2.8279 | 0.1709 | 0.0518 | 30.503 | 0.9973 | |
| RB222 | 32.068 | 0.0193 | 3.5523 | 0.2061 | 0.0222 | 32.227 | 0.9996 | |
| 300 | RR120 | 50.000 | 0.0213 | 1.3612 | 0.2770 | 0.2391 | 49.900 | 0.9999 |
| RY167 | 45.856 | 0.0180 | 10.457 | 0.3515 | 0.0306 | 45.856 | 0.9984 | |
| RB222 | 48.918 | 0.0309 | 12.086 | 0.5965 | 0.0113 | 49.617 | 0.9992 | |
| Adsorbent | Dyes | Qm (mg·g−1) | Ref. |
|---|---|---|---|
| Leather powder | Reactive red 120 Reactive yellow 167 Reactive blue 222 | 167.0 178.9 129.5 | This work |
| Banana peel powder | Reactive black 5 | 49.20 | [8] |
| Congo red | 164.6 | ||
| Silk fibroin powder | Methylene blue | 20.58 | [16] |
| Down powder | Acid brilliant scarlet 3R | 147.7 | [32] |
| Mango seed kernel powder | Methylene blue | 142.9 | [63] |
| Neem tree leaf powder | Methylene blue | 30.66 | [64] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Li, C.; Zhou, S.; Fu, Z.; Wang, Y.; Lyu, P.; Zhang, J.; Liu, X.; Zhang, C.; Xu, W. Utilization of Waste Leather Powders for Highly Effective Removal of Dyes from Water. Polymers 2019, 11, 1786. https://doi.org/10.3390/polym11111786
Xia L, Li C, Zhou S, Fu Z, Wang Y, Lyu P, Zhang J, Liu X, Zhang C, Xu W. Utilization of Waste Leather Powders for Highly Effective Removal of Dyes from Water. Polymers. 2019; 11(11):1786. https://doi.org/10.3390/polym11111786
Chicago/Turabian StyleXia, Liangjun, Chen Li, Sijie Zhou, Zhuan Fu, Yun Wang, Pei Lyu, Jiajing Zhang, Xin Liu, Chunhua Zhang, and Weilin Xu. 2019. "Utilization of Waste Leather Powders for Highly Effective Removal of Dyes from Water" Polymers 11, no. 11: 1786. https://doi.org/10.3390/polym11111786