Effect of the Chain Structure of Self-Emulsifying Polyester Sizing Agent on ILSS of Carbon Fiber/Unsaturated Polyester Resin Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Self-Emulsifying Polyester Sizing Agents
2.3. Sizing Process of Carbon Fibers
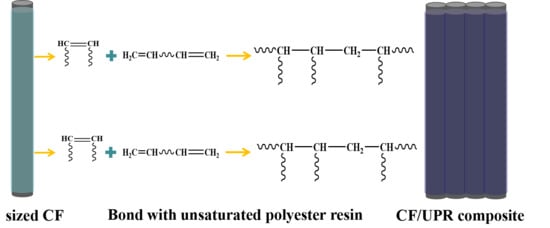
2.4. Preparation of CF/UPR Composite
2.5. Characterization
3. Results and Discussion
3.1. Synthesis of Polyester Sizing Agent
3.2. Emulsion Analyses of Sizing Agent
3.3. Thermal Properties of Sizing Agents
3.4. Surface Energy
3.5. Surface Morphologies of Carbon Fibers
3.6. Interfacial Adhesion of UPR to CFs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soutis, C. Carbon fiber reinforced plastics in aircraft construction. Mat. Sci. Eng. A 2005, 412, 171–176. [Google Scholar] [CrossRef]
- Chand, S. Review Carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Liu, D.; Li, B.; Li, G.; Wang, L.; Yang, X. Tagged and enhanced interface of carbon fiber/epoxy by doping sizing agent with upconversion luminescent nanoparticles. Mater. Lett. 2017, 196, 37–41. [Google Scholar] [CrossRef]
- Martin, A.; Defoort, B.; Kowandy, C.; Coqueret, X. Interfacial layer in high-performance CFRP composites cured out-of-autoclave: Influence of the carbon fiber surface and its graphite-like properties. Compos. Part A 2018, 110, 203–216. [Google Scholar] [CrossRef]
- Jones, A.R.; Cintora, A.; White, S.R.; Sottos, N.R. Autonomic Healing of Carbon Fiber/Epoxy Interfaces. ACS Appl. Mater. Interfaces 2014, 6, 6033–6039. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Chi, Y.; Piao, M.; Chu, J.; Zhang, H.; Li, Z.H.; Wei, W. Effect of Graphene Nanowall Size on the Interfacial Strength of Carbon Fiber Reinforced Composites. Nanomaterials 2018, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Kejing, Y.; Menglei, W.; Wu, J.Q.; Qian, K.; Sun, J.; Lu, X.F. Modification of the Interfacial Interaction between Carbon Fiber and Epoxy with Carbon Hybrid Materials. Nanomaterials 2016, 6, 89. [Google Scholar] [Green Version]
- Yu, J.; Meng, L.; Fan, D.; Zhang, C.H.; Yu, F.; Huang, Y.D. The oxidation of carbon fibers through K2S2O8/AgNO3 system that preserves fiber tensile strength. Compos. Part B 2014, 60, 261–267. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Zhou, Z.; Rahman, A.; Wu, X.F.; Wu, W.; Xu, T.; Fong, H. Fabrication and mechanical properties of hybrid multi-scale epoxy composites reinforced with conventional carbon fiber fabrics surface-attached with electrospun carbon nanofiber mats. Compos. Part B 2013, 44, 1–7. [Google Scholar] [CrossRef]
- Lu, C.R.; Qiu, S.; Lu, X.; Wang, J.; Xiao, L.; Zheng, T.; Wang, X.D.; Zhang, D.X. Enhancing the Interfacial Strength of Carbon Fiber/Poly (ether ether ketone) Hybrid Composites by Plasma Treatments. Polymers 2019, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Netravali, A.N.; Hosur, M.V. Effect of Halloysite Nanotube Incorporation in Epoxy Resin and Carbon Fiber Ethylene/Ammonia Plasma Treatment on Their Interfacial Property. J. Adhes. Sci. Technol. 2012, 26, 1295–1312. [Google Scholar]
- Di, L.Z.; Liu, B.; Song, J.; Shan, D.; Yang, D.A. Effect of chemical etching on the Cu/Ni metallization of poly (ether ether ketone)/carbon fiber composites. Appl. Surf. Sci. 2011, 257, 4272–4277. [Google Scholar] [CrossRef]
- Gu, N.; Li, C.A.; Sun, L.; Liu, Z.H. Controllable fabrication of fiber nano-tips by dynamic chemical etching based on siphon principle. J. Vac. Sci. Technol. B 2004, 22, 2283–2285. [Google Scholar] [CrossRef]
- Rong, H.; Dahmen, K.H.; Garmestani, H.; Yu, M.; Jacob, K.I. Comparison of chemical vapor deposition and chemical grafting for improving the mechanical properties of carbon fiber/epoxy composites with multi-wall carbon nanotubes. J. Mater. Sci. 2013, 48, 4834–4842. [Google Scholar] [CrossRef]
- Lei, X.; Feng, Z.; Liang, H.; Chen, L.; Lan, S.D. Chemical grafting of nano-TiO2 onto carbon fiber via thiol-ene click chemistry and its effect on the interfacial and mechanical properties of carbon fiber/epoxy composites. J. Mater. Sci. 2018, 53, 2594–2603. [Google Scholar]
- Chao, W.; Li, Y.B.; Tong, L.Y.; Song, Q.; Li, K.Z.; Li, J.J.; Peng, Q.Y.; He, X.D.; Wang, R.G.; Jiao, W.C.; et al. The role of grafting force and surface wettability in interfacial enhancement of carbon nanotube/carbon fiber hierarchical composites. Carbon 2014, 69, 239–246. [Google Scholar]
- Zhao, X.; Wen, Y.; Qi, T.; Lai, S.; Gao, L. Original Silk Pre-Oxidation Treatment of Carbon Fiber from Coal Tar Pitch. Adv. Mater. Res. 2014, 989, 194–198. [Google Scholar] [CrossRef]
- Shirazi, M.; de Rooij, M.B.; Talma, A.G.; Noordermeer, J.W.M. Adhesion of RFL-coating aramid fibers to elastomers: The role of elastomer-latex compatibility. J. Adhes. Sci. Technol. 2013, 27, 1886–1898. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Qiu, Y. Influence of aramid fiber moisture regain during atmospheric plasma treatment on aging of treatment effects on surface wettability and bonding strength to epoxy. Appl. Surf. Sci. 2007, 253, 9283–9289. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, S.; Lu, C.; He, S.; An, F. Improved interfacial adhesion in carbon fiber/polyether sulfone composites through an organic solvent-free polyamic acid sizing. Appl. Surf. Sci. 2013, 279, 279–284. [Google Scholar] [CrossRef]
- Giraud, I.; Franceschi-Messant, S.; Perez, E.; Lacabanne, C.; Dantras, E. Preparation of aqueous dispersion of thermoplastic sizing agent for carbon fiber by emulsion/solvent evaporation. Appl. Surf. Sci. 2013, 266, 94–99. [Google Scholar] [CrossRef]
- Liu, J.; Ge, H.; Chen, J.; Wang, D.; Liu, H. The preparation of emulsion type sizing agent for carbon fiber and the properties of carbon fiber/vinyl ester resin composites. J. Appl. Polym. Sci. 2012, 124, 864–872. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Sheltami, R.M.; Ahmad, I.; Abdullah, I.; Dufresne, A. Cellulose nanocrystal: A promising toughening agent for unsaturated polyester nanocomposite. Polymer 2014, 56, 346–357. [Google Scholar] [CrossRef]
- Wu, Z.; Meng, L.; Liu, L.; Jiang, Z.; Xing, L.; Jiang, D.; Huang, Y. Interfacial microstructure and properties of carbon fiber-reinforced unsaturated polyester composites modified with carbon nanotubes. J. Adhes. Sci. Technol. 2013, 28, 444–453. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, Y.; Kang, S.; Zhang, X. Synthesis and Characterization of Novel Epoxy-Modified Waterborne Polyurethanes and their Use in Carbon Fiber Sizing. J. Appl. Polym. Sci. 2012, 125, 3490–3499. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, H.; Chen, L.; Jiang, D.; Weng, L.; Ma, Y.; Li, X.; Zhang, X.; Liu, H.; Wang, N.; et al. Interfacially Reinforced Unsaturated Polyester Carbon Fiber Composites with a Vinyl Ester-Carbon Nanotubes Sizing Agent. Compos. Sci. Technol. 2018, 164, 195–203. [Google Scholar] [CrossRef]
- Jiao, W.; Cai, Y.; Liu, W.; Yang, F.; Jiang, L.; Jiao, W.; Wang, R. Preparation of carbon fiber unsaturated sizing agent for enhancing interfacial strength of carbon fiber/vinyl ester resin composite. Appl. Surf. Sci. 2018, 439, 88–95. [Google Scholar] [CrossRef]
- Shamsuddin, S.R.; Ho, K.K.C.; Ng, P.; Lee, A.F.; Bismarck, A. Synergy of matrix and fibre modification on adhesion between carbon fibres and poly (vinylidene fluoride). Compos. Sci. Technol. 2011, 72, 56–64. [Google Scholar] [CrossRef]
- Saba, N.; Tahir, P.M.; Jawaid, M.A. Review on Potentiality of Nano Filler/Natural Fiber Filled Polymer Hybrid Composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, R.; He, M.; Sun, L.; Wang, C.; Liu, L.; Zhao, L.; Cui, H.; Cao, A. Effect of a multiscale reinforcement by carbon fiber surface treatment with graphene oxide/carbon nanotubes on the mechanical properties of reinforced carbon/carbon composites. Compos. Part A 2016, 90, 433–440. [Google Scholar] [CrossRef]
- Janssen, D.; Palma, R.D.; Verlaak, S.; Heremans, P.; Dehaen, W. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Solid Films 2006, 515, 1433–1438. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, C.X.; Su, X.L.; Wu, G.P.; Wang, X.K. Effect of nano-SiO2 modified emulsion sizing on the interfacial adhesion of carbon fibers reinforced composites. Mater. Lett. 2007, 61, 3601–3604. [Google Scholar] [CrossRef]
















| Sample | Phthalic Anhydride (mol) | Adipic Acid (mol) | Dipropylene Glycol (mol) | Neopentyl Glyol (mol) | Maleic Anhydride (mol) | Mole Ratio |
|---|---|---|---|---|---|---|
| S1 | 0.975 | / | 1.199 | / | 0.3 | 1:1.23:0.31 |
| S2 | / | 0.975 | 1.199 | / | 0.3 | 1:1.23:0.31 |
| S3 | / | 0.975 | / | 1.199 | 0.3 | 1:1.23:0.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Guo, H.; Zhou, H.; Ouyang, X.; Jiang, D.; Li, J.; Guo, Q.; Tang, J.; Yang, C. Effect of the Chain Structure of Self-Emulsifying Polyester Sizing Agent on ILSS of Carbon Fiber/Unsaturated Polyester Resin Composites. Polymers 2019, 11, 1528. https://doi.org/10.3390/polym11091528
Wang Z, Guo H, Zhou H, Ouyang X, Jiang D, Li J, Guo Q, Tang J, Yang C. Effect of the Chain Structure of Self-Emulsifying Polyester Sizing Agent on ILSS of Carbon Fiber/Unsaturated Polyester Resin Composites. Polymers. 2019; 11(9):1528. https://doi.org/10.3390/polym11091528
Chicago/Turabian StyleWang, Zhenyu, Huijun Guo, Hang Zhou, Xinfeng Ouyang, Di Jiang, Jianhua Li, Qipeng Guo, Jun Tang, and Chuncai Yang. 2019. "Effect of the Chain Structure of Self-Emulsifying Polyester Sizing Agent on ILSS of Carbon Fiber/Unsaturated Polyester Resin Composites" Polymers 11, no. 9: 1528. https://doi.org/10.3390/polym11091528