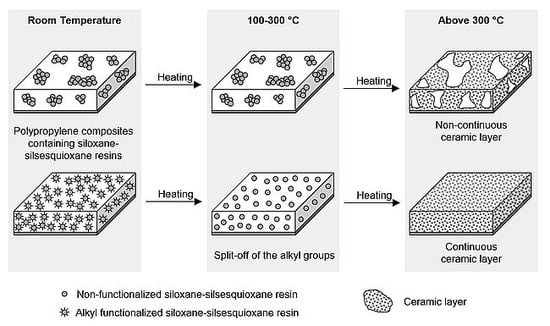
Thermal Stability and Flame Retardancy of Polypropylene Composites Containing Siloxane-Silsesquioxane Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composites Preparation
2.3. Characterization
3. Results and Discussion
3.1. TG Studies
3.2. TG-FTIR Studies
3.3. Fire Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Georlette, P.; Simons, J.; Costa, L. Halogen-containing fire-retardant compounds. In Fire Retardancy of Polymeric Materials; Grand, A.F., Wilkie, C.A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 245–284. ISBN 0-8247-8879-6. [Google Scholar]
- Awad, W.H. Recent Developments in Silicon-Based Flame Retardants. In Fire Retardancy of Polymeric Materials; Wilkie, C.A., Morgan, A.B., Eds.; Taylor and Francis Group, LLC: New York, NY, USA, 2010; pp. 187–206. ISBN 978-1-4200-8399-6. [Google Scholar]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Camino, G. Metal functionalized POSS as fire retardants in polypropylene. Polym. Degrad. Stab. 2006, 91, 2275–2281. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A.; Bottino, P. Synthesis, characterization and thermal stability of new dumbbell-shaped isobutyl-substituted POSSs linked by aromatic bridges. J. Therm. Anal. Calorim. 2014, 117, 243–250. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, S.; Zou, H.; Liang, M. Thermal Stability and Ablation Properties Study of Aluminum Silicate Ceramic Fiber and Acicular Wollastonite Filled Silicone Rubber Composite. J. Appl. Polym. Sci. 2014, 131, 39700. [Google Scholar] [CrossRef]
- Hao, N.; Bohning, M.; Schonhals, A. Dielectric Properties of Nanocomposites Based on Polystyrene and Polyhedral Oligomeric Phenethyl-Silsesquioxanes. Macromolecules 2007, 40, 9672–9679. [Google Scholar] [CrossRef]
- Carroll, J.B.; Waddon, A.J.; Nakade, H.; Rotello, V.M. “Plug and Play” Polymers. Thermal and X-ray Characterizations of Noncovalently Grafted Polyhedral Oligomeric Silsesquioxane (POSS)-Polystyrene Nanocomposites. Macromolecules 2003, 36, 6289–6291. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Wang, S.; Ji, J. Effect of POSS on morphology and properties of poly(2,6-dimethyl-1,4-phenylene oxide)/polyamide 6 blends. Eur. Polym. J. 2009, 45, 2202–2210. [Google Scholar] [CrossRef]
- Jeziórska, R.; Świerz-Motysia, B.; Szadkowska, A.; Marciniec, B.; Maciejewski, H.; Dutkiewicz, M.; Leszczyńska, I. Effect of POSS on morphology, thermal and mechanical properties of polyamide 6. Polimery 2011, 56, 809–816. [Google Scholar]
- Fu, B.X.; Hsiao, B.S.; Pagola, S.; Stephens, P.; White, H.; Rafailovich, M.; Sokolov, J.; Mather, P.T.; Jeon, H.G.; Phillips, S.; et al. Structural development during deformation of polyurethane containing polyhedral oligomeric silsesquioxanes (POSS) molecules. Polymer 2001, 42, 599–611. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A.; Madbouly, S.A.; Otaigbe, J.U. Nanostructured polyurethane/POSS hybrid aqueous dispersions prepared by homogeneous solution polymerization. Macromolecules 2006, 39, 7037–7043. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U. Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films. Prog. Polym. Sci. 2009, 34, 1283–1332. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene–polyhedral oligomeric silsesquioxanes (POSS) nanocomposites. Polymer 2005, 46, 7855–7866. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene metal functionalised POSS nanocomposites: A study by thermogravimetric analysis. Polym. Degrad. Stab. 2006, 91, 1064–1070. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, L.; Zhang, Y.; Zhang, Y.; Yin, N. Isothermal Crystallization Kinetics of Polypropylene/POSS Composites. J. Polym. Sci. Polym. Phys. 2008, 46, 1762–1772. [Google Scholar] [CrossRef]
- Frone, A.N.; Perrin, F.X.; Radovici, C.; Panaitescu, D.M. Influence of branched or un-branched alkyl substitutes of POSS on morphology, thermal and mechanical properties of polyethylene. Compos. Part B Eng. 2013, 50, 98–106. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S.; Simon, G.; Kukaleva, N. Rheological and Viscoelastic Behavior of HDPE/Octamethyl-POSS Nanocomposites. Macromolecules 2006, 39, 1839–1849. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S. Isothermal Crystallization of HDPE/Octamethyl Polyhedral Oligomeric Silsesquioxane Nanocomposites: Role of POSS as a Nanofiller. J. Appl. Polym. Sci. 2007, 105, 978–985. [Google Scholar] [CrossRef]
- Perrin, F.X.; Panaitescu, D.M.; Frone, F.N.; Radovici, C.; Nicolae, C. The influence of alkyl substituents of POSS in polyethylene nanocomposites. Polymer 2013, 54, 2347–2354. [Google Scholar] [CrossRef]
- Barczewski, M.; Sterzyński, T.; Dutkiewicz, M. Thermo-rheological properties and miscibility of linear low-density polyethylene-silsesquioxane nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42825. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Czaja, K.; Szatanik, R.; Szołyga, M.; Dutkiewicz, M.; Marciniec, B. Polypropylene/polyhedral oligomeric silsesquioxane nanocomposites—Study of free volumes, crystallinity degree and mass flow rate. Polimery 2016, 61, 610–615. [Google Scholar] [CrossRef]
- Niemczyk, A.; Adamczyk-Tomiak, K.; Dziubek, K.; Czaja, K.; Rabiej, S.; Szatanik, R.; Dutkiewicz, M. Study of polyethylene nanocomposites with polyhedral oligomeric silsesquioxane nanofillers—From structural characteristics to mechanical properties and processability. Polym. Compos. 2017. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Czaja, K.; Frączek, D.; Piasecki, R.; Adamczyk-Tomiak, K.; Rabiej, S.; Dutkiewicz, M. Study and evaluation of dispersion of polyhedral oligomeric silsesquioxane and silica filler in polypropylene composites. Polym. Compos. 2018. [Google Scholar] [CrossRef]
- Baldi, F.; Bignotti, F.; Fina, A.; Tabuani, D.; Ricco, T. Mechanical Characterization of Polyhedral Oligomeric Silsesquioxane/Polypropylene Blends. J. Appl. Polym. Sci. 2007, 105, 935–943. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jung, C.-H.; Kim, D.-K.; Ganesan, R. Radiation-induced grafting of inorganic particles onto polymer backbone: A new method to design polymer-based nanocomposite. Nucl. Instrum. Methods B 2008, 266, 203–206. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Camino, G. Polypropylene–polysilsesquioxane blends. Eur. Polym. J. 2010, 46, 14–23. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chiou, Y.-D. Crystallization Behavior and Morphological Development of Isotactic Polypropylene Blended with Nanostructured Polyhedral Oligomeric Silsesquioxane Molecules. J. Polym. Sci. Polym. Phys. 2006, 44, 2122–2134. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Peijs, T.; Camino, G. POSS grafting on PPgMA by one-step reactive blending. Polymer 2009, 50, 218–226. [Google Scholar] [CrossRef]
- Hato, M.J.; Ray, S.S.; Luyt, A.S. Nanocomposites Based on Polyethylene and Polyhedral Oligomeric Silsesquioxanes, 1—Microstructure, Thermal and Thermomechanical Properties Macromol. Mater. Eng. 2008, 293, 752–762. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Dutkiewicz, M.; Marciniec, B. Study of thermal properties of polyethylene and polypropylene nanocomposites with long alkyl chain substituted POSS fillers. J. Therm. Anal. Calorim. 2016, 125, 1287–1299. [Google Scholar] [CrossRef]
- Bouza, R.; Barral, L.; Diez, F.J.; Lopez, J.; Montero, B.; Rico, M.; Ramirez, C. Study of thermal and morphological properties of a hybrid system, iPP/POSS. Effect of flame retardance. Compos. Part B Eng. 2014, 58, 566–572. [Google Scholar] [CrossRef]
- Carniato, F.; Fina, A.; Tabuani, D.; Boccaleri, E. Polypropylene containing Ti- and Al-polyhedral oligomeric silsesquioxanes: Crystallization process and thermal properties. Nanotechnology 2008, 19, 475701. [Google Scholar] [CrossRef] [PubMed]
- Butola, B.S.; Joshi, M.; Kumar, S. Hybrid Organic-Inorganic POSS (Polyhedral Oligomeric silsesquioxane)/Polypropylene Nanocomposite Filaments. Fibers Polym. 2010, 11, 1137–1145. [Google Scholar] [CrossRef]
- Carniato, F.; Boccaleri, E.; Marchese, L.; Fina, A.; Tabuani, D.; Camino, G. Synthesis and Characterisation of Metal Isobutylsilsesquioxanes and Their Role as Inorganic–Organic Nanoadditives for Enhancing Polymer Thermal Stability. Eur. J. Inorg. Chem. 2007, 2007, 585–591. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. A kinetic study of the thermal and thermal oxidative degradations of new bridged POSS/PS nanocomposites. Polym. Degrad. Stab. 2013, 98, 2564–2570. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Di Lorenzo, M.L. Polypropylene-based composites containing sorbitol-based nucleating agent and siloxane-silsesquioxane resin. J. Appl. Polym. Sci. 2016, 133, 43476. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Di Lorenzo, M.L. Isotactic polypropylene modified with sorbitol-based derivative and siloxane-silsesquioxane resin. Eur. Polym. J. 2016, 85, 62–71. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Sterzyński, T. Interfacial enhancement of polypropylene composites modified with sorbitol derivatives and siloxane-silsesquioxane resin. AIP Conf. Proc. 2015, 1695, 020049. [Google Scholar] [CrossRef]
- Maciejewski, H.; Dutkiewicz, M.; Byczyński, Ł.; Marciniec, B. Silseskwioksany jako nanonapełniacze. Cz. I. Nanokompozyty z osnową silikonową. Polimery 2012, 57, 535–544. [Google Scholar] [CrossRef]
- Lichtenhan, J.D.; Vu, N.Q.; Carter, J.A. Silsesquioxane-Siloxane Copolymers from Polyhedral Silsesquioxanes. Macromolecules 1993, 26, 2141–2142. [Google Scholar] [CrossRef]
- Liu, Y.R.; Huang, Y.D.; Liu, L. Thermal stability of POSS/methylsilicone nanocomposites. Compos. Sci. Technol. 2007, 67, 2864–2876. [Google Scholar] [CrossRef]
- Gunji, T.; Shioda, T.; Tsuchihira, K.; Seki, H. Preparation and properties of polyhedraloligomeric silsesquioxane–polysiloxane copolymers. Appl. Organomet. Chem. 2010, 24, 545–550. [Google Scholar] [CrossRef]
- Handke, M.; Kowalewska, A. Siloxane and silsesquioxane molecules—Precursors for silicate materials. Spectrochim. Acta A 2011, 79, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Seino, M.; Hayakawa, T.; Ishida, Y.; Kakimoto, M. Synthesis and Characterization of Crystalline Hyperbranched Polysiloxysilane with POSS Groups at the Terminal Position. Macromolecules 2006, 39, 8892–8894. [Google Scholar] [CrossRef]
- Pan, G.; Mark, J.E.; Schaefer, D.W. Synthesis and characterization of fillers of controlled structure based on polyhedral oligomeric silsesquioxane cages and their use in reinforcing siloxane elastomers. J. Polym. Sci. Polym. Phys. 2003, 41, 3314–3323. [Google Scholar] [CrossRef]
- Mantz, R.A.; Jones, P.F.; Chaffee, K.P.; Lichtenhan, J.D.; Gilman, J.W.; Ismail, I.M.K.; Burmeister, M.J. Thermolysis of Polyhedral Oligomeric Silsesquioxane (POSS) Macromers and POSS−Siloxane Copolymers. Chem. Mater. 1996, 8, 1250–1259. [Google Scholar] [CrossRef]
- Ryu, H.S.; Kim, D.G.; Lee, J.C. Polysiloxanes containing polyhedral oligomeric silsesquioxane groups in the side chains; synthesis and properties. Polymer 2010, 51, 2296–2304. [Google Scholar] [CrossRef]
- Zielecka, M.; Bujanowska, E.; Cyruchin, K.; Marciniec, B.; Maciejewski, H.; Dutkiewicz, M. Polysiloxane Composite with Improved Thermal Resistance. PL Patent 393092 A1, 31 May 2013. [Google Scholar]
- Szołyga, M.; Dutkiewicz, M.; Maciejewski, H.; Marciniec, B. Siloxane-Silsesquioxane Resins and Method for Obtaining Them. PL Patent 405584 A1, 29 July 2016. [Google Scholar]
- Szołyga, M.; Dutkiewicz, M.; Maciejewski, H.; Marciniec, B. Siloxane-Silsesquioxane Resins and Method for Obtaining Them. PL Patent 405587 A1, 29 July 2016. [Google Scholar]
- Szołyga, M.; Dutkiewicz, M.; Marciniec, B.; Maciejewski, H. Synthesis of reactive siloxane-silsesquioxane resin. Polimery 2013, 58, 766–771. [Google Scholar] [CrossRef]
- Niemczyk, A.; Czaja, K.; Dziubek, K.; Szołyga, M.; Rabiej, S.; Szatanik, R. Polypropylene composites with functionalized siloxane-silsesquioxane resins as fillers—Comprehensive study of the morphological, structural, thermal and mechanical properties. Polym. Compos. 2018. accepted. [Google Scholar]
- Liu, Y.; Shi, Y.; Zhang, D.; Li, J.; Huang, G. Preparation and thermal degradation behavior of room temperature vulcanized silicone rubber-g-polyhedral oligomeric silsesquioxanes. Polymer 2013, 54, 6140–6149. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Tomer, N.S.; Delor-Jestin, F.; Frezet, L.; Lacoste, J. Oxidation, Chain Scission and Cross-Linking Studies of Polysiloxanes upon Ageings. Open J. Org. Polym. Mater. 2012, 2, 13–22. [Google Scholar] [CrossRef]
- Grassie, N.; Scott, G. Polymer Degradation and Stabilization; Cambridge University Press: Cambridge, UK, 1985; ISBN 0-521-24961-9. [Google Scholar]
- Allen, N.S.; Edge, M. Fundamentals of Polymer Degradation and Stabilization; Elsevier Applied Science: London, UK, 1992; pp. 1–21. ISBN 978-1-85166-773-4. [Google Scholar]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Termochim. Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- Haruvy, Y.; Webber, S.E. Supported Sol-Gel Thin-Film Glasses Embodying Laser Dyes. 3. Optically Clear SiO2 Glass Thin Films Prepared by the Fast Sol-Gel Method. Chem. Mater. 1992, 4, 89–94. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. Synthesis and Characterization of Differently Substituted Phenyl Hepta Isobutyl-Polyhedral Oligomeric Silsesquioxane/Polystyrene Nanocomposites. Polym. Compos. 2014, 35, 151–157. [Google Scholar] [CrossRef]
- Palza, H.; Vergara, R.; Zapata, P. Improving the Thermal Behavior of Poly(propylene) by Addition of Spherical Silica Nanoparticles. Macromol. Mater. Eng. 2010, 295, 899–905. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the use of cone calorimeter data. Polym. Degrad. Stab. 2005, 88, 540–547. [Google Scholar] [CrossRef]
- Song, L.; He, Q.; Hu, Y.; Chen, H.; Liu, L. Study on thermal degradation and combustion behaviors of PC/POSS hybrids. Polym. Degrad. Stab. 2008, 93, 627–639. [Google Scholar] [CrossRef]
- Qu, Y.X.; Chen, Y.; Wang, X.M. Flame Retarded Polymeric Materials; National Defense Industry Press: Beijing, China, 2001. [Google Scholar]
- Duggan, G.J.; Grayson, S.J.; Kumar, S. New fire classifications and fire test methods for the European railway industry. In Flame Retardants 2004, Proceedings of the 11th Conference on Interscience Communications, London, UK, 27–28 January 2004; Interscience Communications Ltd.: London, UK, 2014; pp. 233–240. [Google Scholar]










| Sample | Nitrogen Atmosphere | Air Atmosphere | ||||||
|---|---|---|---|---|---|---|---|---|
| Tmax (°C) | T5 (°C) | T25 (°C) | T50 (°C) | Tmax (°C) | T5 (°C) | T25 (°C) | T50 (°C) | |
| neat PP | 460.9 | 383.7 | 432.6 | 451.5 | 366.9 | 289.1 | 325.4 | 351.8 |
| PP/1%S4SQ-H | 455.3 | 369.1 | 424.5 | 445.2 | 383.3 | 290.9 | 336.8 | 365.3 |
| PP/5%S4SQ-H | - | - | - | - | 382.5 | 292.0 | 336.5 | 365.5 |
| PP/10%S4SQ-H | 449.9 | 370.7 | 419.8 | 440.7 | 380.9 | 282.5 | 332.6 | 363.9 |
| PP/1%S4SQ-8 | 456.8 | 394.1 | 434,2 | 450.0 | 380.6 | 291.6 | 326.8 | 355.7 |
| PP/5%S4SQ-8 | - | - | - | - | 400.6 | 301.5 | 350.7 | 380.2 |
| PP/10%S4SQ-8 | 459.3 | 398.8 | 439.0 | 454.8 | 388.3 | 297.8 | 345.5 | 372.6 |
| PP/1%S4SQ-18 | 459.6 | 405.7 | 441.5 | 454.2 | 404.8 | 298.4 | 347.6 | 379.6 |
| PP/5%S4SQ-18 | - | - | - | - | 409.0 | 298.0 | 346.0 | 376.7 |
| PP/10%S4SQ-18 | 460.5 | 417.7 | 445.6 | 457.0 | 397.7 | 290.3 | 336.2 | 366.2 |
| Sample | TTI(s) | PkHRR (kW/m2) | FPI (m2·s/kW) | MAHRE (kW/m2) | THR (MJ/m2) | EHC (MJ/kg) | Mass Loss (g) |
|---|---|---|---|---|---|---|---|
| PP | 40 | 364 | 0.110 | 154 | 40 | 76 | 15.6 |
| PP/1%S4SQ-H | 41 | 354 | 0.116 | 185 | 47 | 74 | 16.7 |
| PP/5%S4SQ-H | 21 | 500 | 0.042 | 223 | 44 | 77 | 15.9 |
| PP/10%S4SQ-H | 19 | 445 | 0.043 | 214 | 44 | 78 | 16.6 |
| PP/1%S4SQ-8 | 43 | 227 | 0.190 | 115 | 29 | 59 | 14.3 |
| PP/5%S4SQ-8 | 40 | 481 | 0.083 | 215 | 48 | 75 | 17.0 |
| PP/10%S4SQ-8 | - | - | - | - | - | - | - |
| PP/1%S4SQ-18 | 40 | 168 | 0.247 | 68 | 22 | 66 | 12.3 |
| PP/5%S4SQ-18 | 43 | 328 | 0.131 | 160 | 42 | 78 | 15.6 |
| PP/10%S4SQ-18 | 47 | 391 | 0.120 | 183 | 47 | 51 | 16.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Czech-Polak, J.; Oliwa, R.; Lenża, J.; Szołyga, M. Thermal Stability and Flame Retardancy of Polypropylene Composites Containing Siloxane-Silsesquioxane Resins. Polymers 2018, 10, 1019. https://doi.org/10.3390/polym10091019
Niemczyk A, Dziubek K, Sacher-Majewska B, Czaja K, Czech-Polak J, Oliwa R, Lenża J, Szołyga M. Thermal Stability and Flame Retardancy of Polypropylene Composites Containing Siloxane-Silsesquioxane Resins. Polymers. 2018; 10(9):1019. https://doi.org/10.3390/polym10091019
Chicago/Turabian StyleNiemczyk, Arkadiusz, Katarzyna Dziubek, Beata Sacher-Majewska, Krystyna Czaja, Justyna Czech-Polak, Rafał Oliwa, Joanna Lenża, and Mariusz Szołyga. 2018. "Thermal Stability and Flame Retardancy of Polypropylene Composites Containing Siloxane-Silsesquioxane Resins" Polymers 10, no. 9: 1019. https://doi.org/10.3390/polym10091019