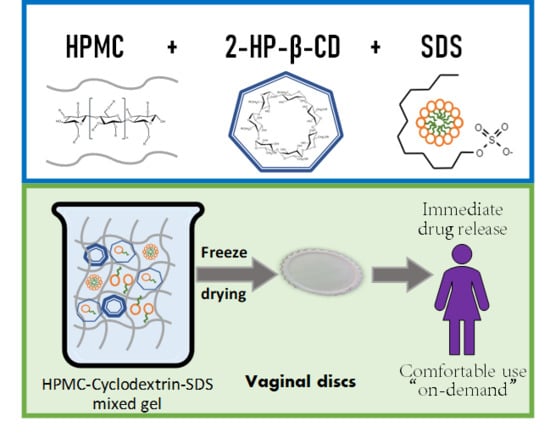
Mucoadhesive Vaginal Discs based on Cyclodextrin and Surfactants for the Controlled Release of Antiretroviral Drugs to Prevent the Sexual Transmission of HIV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gel Preparation
2.3. Gel Characterisation
2.3.1. Viscosity
2.3.2. Penetration and Detachment Work
2.4. Vaginal Disc Preparation
2.5. Vaginal Disc Characterisation
2.5.1. Apparent Density Calculation
2.5.2. Porosity Measurement
2.5.3. Scanning Electron Microscopy (SEM) Micrographs
2.5.4. Mechanical Properties: Deformation and Resistance to Fracture
2.5.5. Swelling Behaviour
2.5.6. Drug Release Studies
2.5.7. Mucoadhesion Time and Force
3. Results and Discussion
3.1. Gel Characterisation
3.1.1. Viscosity
3.1.2. Penetration and Detachment Work
3.2. Vaginal Disc Characterisation
3.2.1. Apparent Density Calculation
3.2.2. Porosity Measurement
3.2.3. Scanning Electron Microscopy (SEM) Micrographs
3.2.4. Mechanical Properties: Deformation and Resistance to Fracture
3.2.5. Swelling Behaviour
3.2.6. Drug Release Studies
3.2.7. Mucoadhesion Time and Force
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Joint Programme on HIV/AIDS (UNAIDS). UNAIDS Data 2019; United Nations Joint Programme on HIV/AIDS (UNAIDS): Geneva, Switzerland, 2019; Available online: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf (accessed on 20 November 2019).
- United Nations Joint Programme on HIV/AIDS (UNAIDS). Fact Sheet—Global AIDS Update 2019; United Nations Joint Programme on HIV/AIDS (UNAIDS): Geneva, Switzerland, 2019; Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 20 November 2019).
- Gupta, S.K.; Nutan, N. Clinical use of vaginal or rectally applied microbicides in patients suffering from HIV/AIDS. HIV AIDS 2013, 5, 295–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antimisiaris, S.G.; Mourtas, S. Recent advances on anti-HIV vaginal delivery systems development. Adv. Drug Deliv. Rev. 2015, 92, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Notario-Pérez, F.; Ruiz-Caro, R.; Veiga-Ochoa, M.D. Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: From past failures to future hopes. Drug Des. Devel. Ther. 2017, 11, 1767–1787. [Google Scholar] [CrossRef] [Green Version]
- das Neves, J.; Nunes, R.; Rodrigues, F.; Sarmento, B. Nanomedicine in the development of anti-HIV microbicides. Adv. Drug Deliv. Rev. 2016, 103, 57–75. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.M.; Tamayo, A.; Rubio, J.; Veiga, M.D. Influence of Chitosan Swelling Behaviour on Controlled Release of Tenofovir from Mucoadhesive Vaginal Systems for Prevention of Sexual Transmission of HIV. Mar. Drugs 2017, 15, 50. [Google Scholar] [CrossRef] [Green Version]
- Cazorla-Luna, R.; Notario-Pérez, F.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Chitosan-Based Mucoadhesive Vaginal Tablets for Controlled Release of the Anti-HIV Drug Tenofovir. Pharmaceutics 2019, 11, E20. [Google Scholar] [CrossRef] [Green Version]
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Bedoya, L.M.; Bermejo, P.; Ruiz-Caro, R.; Veiga, M.D. Dapivirine Bioadhesive Vaginal Tablets Based on Natural Polymers for the Prevention of Sexual Transmission of HIV. Polymers 2019, 11, 483. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Bekker, L.G.; Bukusi, E.; Hellstrӧm, E.; Kotze, P.; Louw, C.; Martinson, F.; Masenga, G.; Montgomery, E.; Ndaba, N.; et al. Safety, Acceptability and Adherence of Dapivirine Vaginal Ring in a Microbicide Clinical Trial Conducted in Multiple Countries in Sub-Saharan Africa. PLoS ONE 2016, 11, e0147743. [Google Scholar] [CrossRef] [Green Version]
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Mgodi, N.M.; Matovu Kiweewa, F.; Nair, G.; Mhlanga, F.; et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N. Engl. J. Med. 2016, 375, 2121–2132. [Google Scholar] [CrossRef]
- Akil, A.; Devlin, B.; Cost, M.; Rohan, L.C. Increased Dapivirine tissue accumulation through vaginal film codelivery of dapivirine and Tenofovir. Mol. Pharm. 2014, 11, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Gupta, K.M.; Fabian, J.; Albright, T.H.; Kiser, P.F. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 2010, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Woodsong, C.; Holt, J.D. Acceptability and preferences for vaginal dosage forms intended for prevention of HIV or HIV and pregnancy. Adv. Drug Deliv. Rev. 2015, 92, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Martín-Illana, A.; Cazorla-Luna, R.; Notario-Pérez, F.; Bedoya, L.M.; Ruiz-Caro, R.; Veiga, M.D. Freeze-dried bioadhesive vaginal bigels for controlled release of Tenofovir. Eur. J. Pharm. Sci. 2019, 127, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D. Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse. Pharmaceutics 2019, 11, 232. [Google Scholar] [CrossRef] [Green Version]
- Woolfson, A.D.; Umrethia, M.L.; Kett, V.L.; Malcolm, R.K. Freeze-dried, mucoadhesive system for vaginal delivery of the HIV microbicide, dapivirine: Optimisation by an artificial neural network. Int. J. Pharm. 2010, 388, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Veiga, M.D.; Ruiz-Caro, R.; Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R. Polymer Gels in Vaginal Drug Delivery Systems. In Polymer Gels. Gels Horizons: From Science to Smart Materials; Thakur, V., Thakur, M., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Hiorth, M.; Nilsen, S.; Tho, I. Bioadhesive mini-tablets for vaginal drug delivery. Pharmaceutics 2014, 6, 494–511. [Google Scholar] [CrossRef] [Green Version]
- Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Lupuleasa, D. Cellulose-Derivatives-Based Hydrogels as Vehicles for Dermal and Transdermal Drug Delivery. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Shokri, J.; Adibkia, K. Application of Cellulose and Cellulose Derivatives in Pharmaceutical Industries. In Cellulose— Medical, Pharmaceutical and Electronic Applications; Van de Ven, T., Godbout, L., Eds.; IntechOpen: London, UK, 2013. [Google Scholar]
- Gafitanu, C.A.; Filip, D.; Cernatescu, C.; Rusu, D.; Tuchilus, C.G.; Macocinschi, D.; Zaltariov, M.F. Design, Preparation and Evaluation of HPMC-Based PAA or SA Freeze-Dried Scaffolds for Vaginal Delivery of Fluconazole. Pharm. Res. 2017, 34, 2185–2196. [Google Scholar] [CrossRef]
- Marcos, X.; Pérez-Casas, S.; Llovo, J.; Concheiro, A.; Alvarez-Lorenzo, C. Poloxamer-hydroxyethyl cellulose-α-cyclodextrin supramolecular gels for sustained release of griseofulvin. Int. J. Pharm. 2016, 500, 11–19. [Google Scholar] [CrossRef]
- Veiga, M.D.; Ahsan, F. Study of Surfactants/β-Cyclodextrin Interactions over Mequitazine Dissolution. Drug Dev. Ind. Pharm. 1997, 23, 721–725. [Google Scholar] [CrossRef]
- Ruiz-Caro, R.; Veiga-Ochoa, M.D. Characterization and dissolution study of chitosan freeze-dried systems for drug controlled release. Molecules 2009, 14, 4370–4386. [Google Scholar] [CrossRef] [PubMed]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.M.; Peña, J.; Veiga, M.D. Development of mucoadhesive vaginal films based on HPMC and zein as novel formulations to prevent sexual transmission of HIV. Int. J. Pharm. 2019, 570, 118643. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Oh, C.M.; Heng, P.W.; Chan, L.W. A study on the impact of hydroxypropyl methylcellulose on the viscosity of PEG melt suspensions using surface plots and principal component analysis. AAPS PharmSciTech 2015, 16, 466–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuğcu-Demiröz, F. Vaginal Delivery of Benzydamine Hydrochloride through Liposomes Dispersed in Mucoadhesive Gels. Chem. Pharm. Bull 2017, 65, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Karakatsani, M.; Dedhiya, M.; Plakogiannis, F.M. The effect of permeation enhancers on the viscosity and the release profile of transdermal hydroxypropyl methylcellulose gel formulations containing diltiazem HCl. Drug Dev. Ind. Pharm. 2010, 36, 1195–1206. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Cheng, L.; Gu, Z.; Hong, Y.; Kowalczyk, A. Improving the performance of starch-based wood adhesive by using sodium dodecyl sulfate. Carbohydr. Polym. 2014, 99, 579–583. [Google Scholar] [CrossRef]
- Tölle, A.; Meier, W.; Rüdiger, M.; Hofmann, K.P.; Rüstow, B. Effect of cholesterol and surfactant protein B on the viscosity of phospholipid mixtures. Chem. Phys. Lipids 2002, 114, 159–168. [Google Scholar] [CrossRef]
- Lin, S.Y.; Yang, J.C. Effect of beta-cyclodextrin on the in vitro permeation rate and in vivo rectal absorption of acetaminophen hydrogel preparations. Pharm. Acta Helv. 1990, 65, 262–268. [Google Scholar] [PubMed]
- Pose-Vilarnovo, B.; Rodríguez-Tenreiro, C.; Rosa dos Santos, J.F.; Vázquez-Doval, J.; Concheiro, A.; Alvarez-Lorenzo, C.; Torres-Labandeira, J.J. Modulating drug release with cyclodextrins in hydroxypropyl methylcellulose gels and tablets. J. Control Release 2004, 94, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Bozkir, A.; Denli, Z.F.; Basaran, B. Effect of hydroxypropyl-beta-cyclodextrin on the solubility, stability and in-vitro release of ciprofloxacin for ocular drug delivery. Acta Pol. Pharm. 2012, 69, 719–724. [Google Scholar] [PubMed]
- Li, Z.; Li, H.; Wang, C.; Xu, J.; Singh, V.; Chen, D.; Zhang, J. Sodium dodecyl sulfate/β-cyclodextrin vesicles embedded in chitosan gel for insulin delivery with pH-selective release. Acta Pharm. Sin. B 2016, 6, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brocos, P.; Díaz-Vergara, N.; Banquy, X.; Pérez-Casas, S.; Costas, M.; Piñeiro, A. Similarities and differences between cyclodextrin-sodium dodecyl sulfate host-guest complexes of different stoichiometries: Molecular dynamics simulations at several temperatures. J. Phys. Chem. B 2010, 114, 12455–12467. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Djokic, J. Texture profile analysis of bioadhesive polymeric semisolids: Mechanical characterisation and investigation of interactions between formulation components. J. Appl. Poly. Sci. 1996, 61, 2229–2234. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Li, L.; Li, B.; Zhang, X.; Su, J. Mechanical, rheological and release behaviors of a poloxamer 407/ poloxamer 188/carbopol 940 thermosensitive composite hydrogel. Molecules 2013, 18, 12415–12425. [Google Scholar] [CrossRef]
- Garcia, M.T.J.; de Paula Freitas, C.; Graciano, T.B.; Coutinho, T.S.; Cressoni, C.B.; de Lima Pereira, S.A.; Shimano, M.M. Chitosan-based mucoadhesive gel for oral mucosal toluidine blue O delivery: The influence of a non-ionic surfactant. Photodiagnosis Photodyn. Ther. 2017, 20, 48–54. [Google Scholar] [CrossRef]
- Devi, S.; Williams, D.R. Density dependent mechanical properties and structures of a freeze dried biopharmaceutical excipient--sucrose. Eur. J. Pharm. Biopharm. 2014, 88, 492–501. [Google Scholar] [CrossRef]
- Wilson, L.D.; Mohamed, M.H.; Headley, J.V. Surface area and pore structure properties of urethane-based copolymers containing β-cyclodextrin. J. Colloid Interface Sci. 2011, 357, 215–222. [Google Scholar] [CrossRef]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly(AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Jayakumar, R. Chitosan-graft-beta-cyclodextrin scaffolds with controlled drug release capability for tissue engineering applications. Int. J. Biol. Macromol. 2009, 44, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Dakwar, G.R.; Zagato, E.; Lechanteur, A.; Remaut, K.; Evrard, B.; Braeckmans, K.; Piel, G. Freeze-dried mucoadhesive polymeric system containing pegylated lipoplexes: Towards a vaginal sustained released system for siRNA. J. Control Release 2016, 236, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymańska, E.; Winnicka, K.; Amelian, A.; Cwalina, U. Vaginal chitosan tablets with clotrimazole-design and evaluation of mucoadhesive properties using porcine vaginal mucosa, mucin and gelatine. Chem. Pharm. Bull. 2014, 62, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perioli, L.; Ambrogi, V.; Pagano, C.; Massetti, E.; Rossi, C. New solid mucoadhesive systems for benzydamine vaginal administration. Colloids Surf. B Biointerfaces 2011, 84, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Dharmala, K.; Lee, C.H. The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system. Int. J. Pharm. 2006, 309, 139–145. [Google Scholar] [CrossRef]
- Machado, A.; Cunha-Reis, C.; Araújo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; das Neves, J.; Sarmento, B. Development and in vivo safety assessment of tenofovir-loaded nanoparticles-in-film as a novel vaginal microbicide delivery system. Acta Biomater. 2016, 44, 332–340. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Marzec, A.; Mieszkowska, A.; Lenart, A. Structure influence on mechanical and acoustic properties of freeze-dried gels obtained with the use of hydrocolloids. J. Texture Stud. 2017, 48, 131–142. [Google Scholar] [CrossRef]
- Gennari, C.G.M.; Sperandeo, P.; Polissi, A.; Minghetti, P.; Cilurzo, F. Lysozyme Mucoadhesive Tablets Obtained by Freeze-Drying. J. Pharm. Sci. 2019, 108, 3667–3674. [Google Scholar] [CrossRef]
- Salmaso, S.; Semenzato, A.; Bersani, S.; Matricardi, P.; Rossi, F.; Caliceti, P. Cyclodextrin/PEG based hydrogels for multi-drug delivery. Int. J. Pharm. 2007, 345, 42–50. [Google Scholar] [CrossRef]
- Yi, S.; Zheng, J.; Lv, P.; Zhang, D.; Zheng, X.; Zhang, Y.; Liao, R. Controlled Drug Release from Cyclodextrin-Gated Mesoporous Silica Nanoparticles Based on Switchable Host-Guest Interactions. Bioconjug. Chem. 2018, 29, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Akil, A.; Agashe, H.; Dezzutti, C.S.; Moncla, B.J.; Hillier, S.L.; Devlin, B.; Shi, Y.; Uranker, K.; Rohan, L.C. Formulation and characterization of polymeric films containing combinations of antiretrovirals (ARVs) for HIV prevention. Pharm. Res. 2015, 32, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.J.; Desjardins, D.; Dereuddre-Bosquet, N.; Brochard, P.; Perrot, L.; Pruvost, A.; Le Grand, R.; Lagatie, O.; Vanhooren, L.; Feyaerts, M.; et al. Pre-clinical development of a combination microbicide vaginal ring containing dapivirine and darunavir. J. Antimicrob. Chemother. 2014, 69, 2477–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regev, G.; Patel, S.K.; Moncla, B.J.; Twist, J.; Devlin, B.; Rohan, L.C. Novel Application of Hot Melt Extrusion for the Manufacturing of Vaginal Films Containing Microbicide Candidate Dapivirine. AAPS PharmSciTech 2019, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Welsh, N.R.; Malcolm, R.K.; Devlin, B.; Boyd, P. Dapivirine-releasing vaginal rings produced by plastic freeforming additive manufacturing. Int. J. Pharm. 2019, 572, 118725. [Google Scholar] [CrossRef] [PubMed]
- Inácio, Â.S.; Mesquita, K.A.; Baptista, M.; Ramalho-Santos, J.; Vaz, W.L.; Vieira, O.V. In vitro surfactant structure-toxicity relationships: Implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS ONE 2011, 6, e19850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faisal, Z.; Garai, E.; Csepregi, R.; Bakos, K.; Fliszár-Nyúl, E.; Szente, L.; Balázs, A.; Cserháti, M.; Kőszegi, T.; Urbányi, B.; et al. Protective effects of beta-cyclodextrins vs. zearalenone-induced toxicity in HeLa cells and Tg(vtg1:mCherry) zebrafish embryos. Chemosphere 2020, 240, 124948. [Google Scholar] [CrossRef]
- Singh, S.; Nwabor, O.F.; Ontong, J.C.; Kaewnopparat, N.; Voravuthikunchai, S.P. Characterization of a novel, co-processed bio-based polymer, and its effect on mucoadhesive strength. Int. J. Biol. Macromol. 2019, 145, 865–875. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, X.; Zhai, D.; Gao, F.; Guo, T.; Li, W.; Hao, S.; Ji, J.; Wang, B. Development of keratin nanoparticles for controlled gastric mucoadhesion and drug release. J. Nanobiotechnology 2018, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Li, J.; Kong, M.; Liu, Y.; Cheng, X.J.; Li, Y.; Park, H.J.; Chen, X.G. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 439–447. [Google Scholar] [CrossRef]
- Vincent, K.L.; Moss, J.A.; Marzinke, M.A.; Hendrix, C.W.; Anton, P.A.; Pyles, R.B.; Guthrie, K.M.; Dawson, L.; Olive, T.J.; Butkyavichene, I.; et al. Safety and pharmacokinetics of single, dual, and triple antiretroviral drug formulations delivered by pod-intravaginal rings designed for HIV-1 prevention: A Phase I trial. PLoS Med. 2018, 15, e1002655. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, L.; Galante, J.; Nunes, R.; Sarmento, B.; das Neves, J. Pharmaceutical Vehicles for Vaginal and Rectal Administration of Anti-HIV Microbicide Nanosystems. Pharmaceutics 2019, 11, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Bedoya, L.M.; Tamayo, A.; Rubio, R.; Ruiz-Caro, J.; Veiga, M.D. Vaginal Polyelectrolyte Layer-by-Layer Films Based on Chitosan Derivatives and Eudragit® S100 for pH Responsive Release of Tenofovir. Mar. Drugs 2020, 18, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Group | Batch | Hydroxypropylmethyl Cellulose | 2-Hydroxypropyl-β-Cyclodextrin | Sodium Dodecyl Sulphate | Polysorbate 60 | Tenofovir | Dapivirine |
|---|---|---|---|---|---|---|---|
| Unloaded | H | 80 | |||||
| HC | 80 | 400 | |||||
| HS | 80 | 400 | |||||
| HSC | 80 | 400 | 400 | ||||
| HT | 80 | 400 | |||||
| HTC | 80 | 400 | 400 | ||||
| TFV-loaded | H-TFV | 80 | 10 | ||||
| HC-TFV | 80 | 400 | 10 | ||||
| HS-TFV | 80 | 400 | 10 | ||||
| HSC-TFV | 80 | 400 | 400 | 10 | |||
| HT-TFV | 80 | 400 | 10 | ||||
| HTC-TFV | 80 | 400 | 400 | 10 | |||
| DPV-loaded | H-DPV | 80 | 5 | ||||
| HC-DPV | 80 | 400 | 5 | ||||
| HS-DPV | 80 | 400 | 5 | ||||
| HSC-DPV | 80 | 400 | 400 | 5 | |||
| HT-DPV | 80 | 400 | 5 | ||||
| HTC-DPV | 80 | 400 | 400 | 5 |
| Reference Group | Problem Group | p Value (α = 0.05) | |
|---|---|---|---|
| Penetration Work | Detachment Work | ||
| Unloaded | TFV-loaded | 0.2083 | 0.4193 |
| Unloaded | DPV-loaded | 0.1798 | 0.8829 |
| TFV-loaded | DPV-loaded | 0.2647 | 0.3584 |
| H | HC | 0.0151 | 0.0547 |
| H | HS | 0.0028 | 0.0142 |
| H | HSC | 0.0016 | 0.2025 |
| H | HT | 0.0134 | 0.0124 |
| H | HTC | 0.0640 | 0.0138 |
| HC | HS | 0.3661 | 0.0013 |
| HC | HSC | 0.0112 | 0.0120 |
| HC | HT | 0.0495 | 0.0015 |
| HC | HTC | 0.1234 | 0.0016 |
| HS | HSC | 0.0176 | 0.0011 |
| HS | HT | 0.0328 | 0.9020 |
| HS | HTC | 0.0235 | 0.0654 |
| HSC | HT | 0.2105 | 0.0007 |
| HSC | HTC | 0.0197 | 0.0007 |
| HT | HTC | 0.0107 | 0.0052 |
| Reference | Problem | f2 Value | ||
|---|---|---|---|---|
| TFV-loaded SVF | DPV-loaded SVF + SLS | DPV-loaded SVF | ||
| H | HC | 37.0 | 65.1 | 72.5 |
| H | HS | 36.7 | 27.5 | 15.3 |
| H | HSC | 61.0 | 27.8 | 14.0 |
| H | HT | 37.4 | 28.2 | 19.3 |
| H | HTC | 51.0 | 34.2 | 30.1 |
| HC | HS | 23.6 | 24.7 | 15.7 |
| HC | HSC | 31.9 | 24.9 | 14.5 |
| HC | HT | 24.0 | 25.1 | 20.6 |
| HC | HTC | 51.0 | 30.4 | 32.5 |
| HS | HSC | 36.3 | 43.3 | 43.3 |
| HS | HT | 96.6 | 56.1 | 33.1 |
| HS | HTC | 26.9 | 33.6 | 20.7 |
| HSC | HT | 37.0 | 60.0 | 36.9 |
| HSC | HTC | 41.5 | 46.9 | 22.8 |
| HT | HTC | 27.4 | 44.8 | 38.3 |
| Reference Group | Problem Group | p-Value |
|---|---|---|
| Unloaded | TFV-loaded | 0.5441 |
| Unloaded | DPV-loaded | 0.6698 |
| TFV-loaded | DPV-loaded | 0.1095 |
| H | HC | 0.2851 |
| H | HS | 0.1769 |
| H | HSC | 0.8837 |
| H | HT | 0.2778 |
| H | HTC | 0.1916 |
| HC | HS | 0.0364 |
| HC | HSC | 0.4117 |
| HC | HT | 0.4179 |
| HC | HTC | 0.3869 |
| HS | HSC | 0.0577 |
| HS | HT | 0.0047 |
| HS | HTC | 0.0008 |
| HSC | HT | 0.0492 |
| HSC | HTC | 0.1563 |
| HT | HTC | 0.9514 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; María-Dolores, V. Mucoadhesive Vaginal Discs based on Cyclodextrin and Surfactants for the Controlled Release of Antiretroviral Drugs to Prevent the Sexual Transmission of HIV. Pharmaceutics 2020, 12, 321. https://doi.org/10.3390/pharmaceutics12040321
Notario-Pérez F, Martín-Illana A, Cazorla-Luna R, Ruiz-Caro R, Tamayo A, Rubio J, María-Dolores V. Mucoadhesive Vaginal Discs based on Cyclodextrin and Surfactants for the Controlled Release of Antiretroviral Drugs to Prevent the Sexual Transmission of HIV. Pharmaceutics. 2020; 12(4):321. https://doi.org/10.3390/pharmaceutics12040321
Chicago/Turabian StyleNotario-Pérez, Fernando, Araceli Martín-Illana, Raúl Cazorla-Luna, Roberto Ruiz-Caro, Aitana Tamayo, Juan Rubio, and Veiga María-Dolores. 2020. "Mucoadhesive Vaginal Discs based on Cyclodextrin and Surfactants for the Controlled Release of Antiretroviral Drugs to Prevent the Sexual Transmission of HIV" Pharmaceutics 12, no. 4: 321. https://doi.org/10.3390/pharmaceutics12040321