Protein-Pacing from Food or Supplementation Improves Physical Performance in Overweight Men and Women: The PRISE 2 Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Exercise Training
2.4. Laboratory Testing Procedures
2.5. Body Weight and Composition
2.6. Dietary Intake and Feelings of Hunger and Satiety
2.7. Physical Performance Assessments
2.8. Cardiometabolic Biomarkers
2.9. Resting Energy Expenditure (REE), Heart Rate, and Blood Pressure
2.10. Internet-Based Healthy Lifestyle
2.11. Statistical Analysis
3. Results
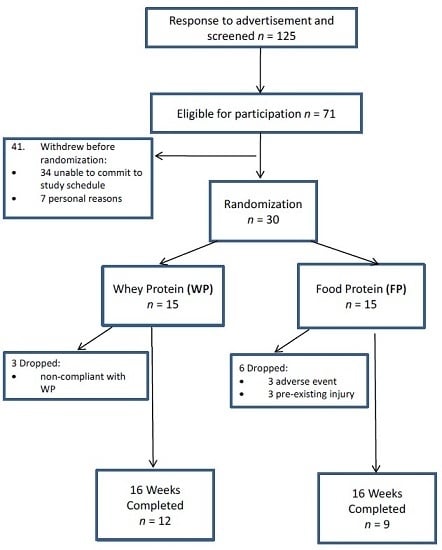
3.1. Participants and Compliance
3.2. Physical Performance Assessments
3.3. Body Weight and Composition and Resting Energy Expenditure
3.4. Cardiometabolic Markers
3.5. Dietary Intake and Self-Reported Feelings of Hunger, Desire to Eat, and Satiety
4. Discussion
4.1. Physical Performance
4.2. Body Composition
4.3. Cardiometabolic Biomarkers
4.4. Satiation and Hunger Ratings, and Dietary Intake
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PRISE | protein-pacing, resistance, interval, stretching, endurance training |
| WP | whey protein |
| FP | food protein |
| VAT | visceral adipose tissue |
| TC | total cholesterol |
| HDL-C | high density lipoprotein cholesterol |
| LDL-C | low density lipoprotein cholesterol |
| TRG | triglycerides |
| GLU | glucose |
| REE | resting energy expenditure |
References
- Goisser, S.; Kemmler, W.; Porzel, S.; Volkert, D.; Sieber, C.C.; Bollheimer, L.C.; Freiberger, E. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons—A narrative review. Clin. Interv. Aging 2015, 10, 1267–1282. [Google Scholar] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80 (Suppl. S1), A8–A15. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.A.; Westerterp-Plantenga, M.S. Protein diets, body weight loss and weight maintenance. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Ormsbee, M.J.; Gentile, C.L.; Nindl, B.C.; Brestoff, J.R.; Ruby, M. Increased protein intake and meal frequency reduces abdominal fat during energy balance and energy deficit. Obesity 2013, 21, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Ramadas, A.; Quek, K.F.; Chan, C.K.; Oldenburg, B. Web-based interventions for the management of type 2 diabetes mellitus: A systematic review of recent evidence. Int. J. Med. Inform. 2011, 80, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.K.; Atlantis, E.; Singh, M.A.F. Multi-modal exercise programs for older adults. Age Ageing 2007, 36, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Marzolini, S.; Oh, P.I.; Brooks, D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: A meta-analysis. Eur. J. Prev. Cardiol. 2012, 19, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, C.G.; Seo, T.B.; Kim, H.G.; Yoon, S.J. Effects of 8-week combined training on body composition, isokinetic strength, and cardiovascular disease risk factors in older women. Aging Clin. Exp. Res. 2015, 27, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sillanpaa, E.; Hakkinen, A.; Nyman, K.; Mattila, M.; Cheng, S.; Karavirta, L.; Laaksonen, D.E.; Huuhka, N.; Kraemer, W.J.; Hakkinen, K. Body composition and fitness during strength and/or endurance training in older men. Med. Sci. Sports Exerc. 2008, 40, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Boutcher, S.H. High-intensity intermittent exercise and fat loss. J. Obes. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Aladro-Gonzalvo, A.R.; Machado-Diaz, M.; Moncada-Jimenez, J.; Hernandez-Elizondo, J.; Araya-Vargas, G. The effect of Pilates exercises on body composition: A systematic review. J. Bodyw. Mov. Ther. 2012, 16, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Telles, S.; Naveen, V.K.; Balkrishna, A.; Kumar, S. Short term health impact of a yoga and diet change program on obesity. Med. Sci. Monit. 2010, 16, CR35–CR40. [Google Scholar] [PubMed]
- Arciero, P.J.; Baur, D.; Connelly, S.; Ormsbee, M.J. Timed-daily ingestion of whey protein and exercise training reduces visceral adipose tissue mass and improves insulin resistance: The PRISE study. J. Appl. Physiol. 2014, 117, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.; van Loon, L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Radavelli-Bagatini, S.; Hagger, M.; Ellis, V. Comparative effects of whey and casein proteins on satiety in overweight and obese individuals: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.J.; Stote, K.S.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Clevidence, B.A. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J. Nutr. 2011, 141, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.M.; Lowery, R.P.; Wilson, J.M.; Purpura, M.; de Souza, E.O.; Wilson, S.M.; Kalman, D.S.; Dudeck, J.E.; Jager, R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr. J. 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Peacock, C.A.; Ellerbroek, A.; Fromhoff, B.; Silver, T. The effects of consuming a high protein diet (4.4 g/kg/day) on body composition in resistance-trained individuals. J. Int. Soc. Sports Nutr. 2014, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Gentile, C.L.; Pressman, R.; Everett, M.; Ormsbee, M.J.; Martin, J.; Santamore, J.; Gorman, L.; Fehling, P.C.; Vukovich, M.D.; et al. Moderate protein intake improves total and regional body composition and insulin sensitivity in overweight adults. Metabolism 2008, 57, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Gentile, C.L.; Martin-Pressman, R.; Ormsbee, M.J.; Everett, M.; Zwicky, L.; Steele, C.A. Increased dietary protein and combined high intensity aerobic and resistance exercise improves body fat distribution and cardiovascular risk factors. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 373–392. [Google Scholar] [PubMed]
- Pasiakos, S.M.; Austin, K.G.; Lieberman, H.R.; Askew, E.W. Efficacy and safety of protein supplements for U.S. Armed Forces personnel: Consensus statement. J. Nutr. 2013, 143, 1811S–1814S. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cribb, P.J.; Williams, A.D.; Carey, M.F.; Hayes, A. The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Lauwers-Cances, V.; Cristini, C.; van Kan, G.A.; Janssen, I.; Morley, J.E.; Vellas, B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: The EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am. J. Clin. Nutr. 2009, 89, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Bangsbo, J.; Jensen, J.; Bibby, B.M.; Madsen, K. Effect of whey protein hydrolysate on performance and recovery of top-class orienteering runners. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Hartman, J.W.; Wilkinson, S.B. Dietary protein to support anabolism with resistance exercise in young men. J. Am. Coll. Nutr. 2005, 24, 134S–139S. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; McGregor, R.A.; D’Souza, R.F.; Thorstensen, E.B.; Markworth, J.F.; Fanning, A.C.; Poppitt, S.D.; Cameron-Smith, D. Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Nutrients 2015, 7, 8685–8699. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.B.; Fox, C.D.; Ferguson, B.S.; Pascoe, C.A.; Healy, J.C.; McAdam, J.S.; Lockwood, C.M.; Roberts, M.D. Effects of protein type and composition on postprandial markers of skeletal muscle anabolism, adipose tissue lipolysis, and hypothalamic gene expression. J. Int. Soc. Sports Nutr. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Choi, M.D.; Medlin, J.K.; Geyer, G.H.; Trantham, L.H.; Dubis, G.S.; Hickner, R.C. Regulation of fat metabolism during resistance exercise in sedentary lean and obese men. J. Appl. Physiol. 2009, 106, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Weinheimer, E.M.; Conley, T.B.; Kobza, V.M.; Sands, L.P.; Lim, E.; Janle, E.M.; Campbell, W.W. Whey protein supplementation does not affect exercise training-induced changes in body composition and indices of metabolic syndrome in middle-aged overweight and obese adults. J. Nutr. 2012, 142, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Chatterjee, T.K.; Tang, Y.; Hui, D.Y.; Weintraub, N.L. Proinflammatory phenotype of perivascular adipocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Abranches, M.V.; Oliveira, F.C.; Conceicao, L.L.; Peluzio, M.D. Obesity and diabetes: The link between adipose tissue dysfunction and glucose homeostasis. Nutr. Res. Rev. 2015, 28, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, S.; Kadamkode, V.; Mahammed, R.; Doraiswami, C.; Banerjee, G. Adiponectin and IL-6: Mediators of inflammation in progression of healthy to type 2 diabetes in Indian population. Adipocyte 2014, 3, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Rawal, S.R.; Baur, D.A.; Kinsey, A.W.; Elam, M.L.; Spicer, M.T.; Fischer, N.T.; Madzima, T.A.; Thomas, D.D. The effects of a multi-ingredient dietary supplement on body composition, adipokines, blood lipids, and metabolic health in overweight and obese men and women: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Kopaliani, I.; Jannasch, A.; Mund, C.; Todorov, V.; Henle, T.; Deussen, A. Antihypertensive and cardioprotective effects of the dipeptide isoleucine-tryptophan and whey protein hydrolysate. Acta Physiol. 2015, 215, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Ward, E.; Holst, J.J.; Astrup, A.; Ormsbee, M.J.; Connelly, S.; Arciero, P.J. Resistant starch and protein intake enhances fat oxidation and feelings of fullness in lean and overweight/obese women. Nutr. J. 2015, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie-Shalders, K.L.; Byrne, N.M.; Slater, G.J.; King, N.A. The effect of a whey protein supplement dose on satiety and food intake in resistance training athletes. Appetite 2015, 92, 178–184. [Google Scholar] [CrossRef] [PubMed]




| Variable | WP | FP |
|---|---|---|
| Sex (M/F) | 4/5 | 7/5 |
| Age (year) | 48 ± 4 | 52 ± 1 |
| Height (cm) | 173 ± 3 | 172 ± 3 |
| Weight (kg) | 96 ± 3 | 97 ± 5 |
| Body mass index | 32 ± 2 | 33 ± 1 |
| Variable | WP | FP | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Δ % | Pre | Post | Δ % | |
| Body Mass (kg) | 95.8 ± 6.1 | 90.9 ± 5.5 a | −4.8 ± 1.2 | 96.9 ± 4.8 | 92.2 ± 4.4 a | −4.8 ± 0.9 |
| Waist (cm) | 105.2 ± 3.7 | 94.3 ± 3.0 a | −10.1 ± 1.9 | 104.7 ± 3.1 | 96.3 ± 3.2 a | −7.9 ± 1.3 |
| Fat Mass (kg) | 35.8 ± 2.9 | 31.7 ± 2.6 a | −10.9 ± 2.3 | 38.4 ± 2.8 | 34.1 ± 2.8 a | −11.9 ± 1.8 |
| % Fat Mass | 38.8 ± 1.9 | 36.2 ± 1.8 a | −6.8 ± 1.6 | 41.4 ± 2.4 | 38.5 ± 2.6 a | −7.5 ± 1.2 |
| AbFat Mass (kg) | 5.1 ± 0.4 | 4.2 ± 0.3 a | −15.3 ± 4.7 | 5.5 ± 0.4 | 4.7 ± 0.4 a | −15.9 ± 2.8 |
| VAT (g) | 1232.6 ± 219.9 | 884. 9 ± 173.3 a | −29.9 ± 5.6 | 1816.8 ± 262.2 | 1498.2 ± 221.9 a | −18.1 ± 4.8 |
| Variable | WP | FP | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| HR (beats/min) | 63.3 ± 4.0 | 61.1 ± 2.1 | 64.8 ± 2.8 | 61.0 ± 2.3 |
| SBP (mmHg) | 130.4 ± 5.0 | 116.0 ± 4.1 a | 124.3 ± 3.3 | 119.2 ± 2.9 a |
| DBP (mmHg) | 83.6 ± 2.7 | 80.3 ± 2.3 | 84.8 ± 2.4 | 84.0 ± 2.1 |
| TGL (mg/dL) | 171.8 ± 29.8 | 123.2 ± 27.0 a | 94.2 ± 8.5 * | 104.4 ± 12.8 # |
| Cholesterol (mg/dL) | 192.2 ± 20.0 | 151.3 ± 10.9 a | 197.8 ± 8.3 | 166.3 ± 5.0 a |
| HDL (mg/dL) | 42. 6 ± 4.0 | 46.1 ± 4.6 | 52.8 ± 4.6 * | 50.0 ± 4.7 # |
| LDL (mg/dL) | 115. 2 ± 20.1 | 92.0 ± 9.6 a | 126.2 ±7.8 | 98.9 ± 5.1 a |
| GLU (mg/dL) | 97. 0 ± 4.6 | 93.6 ± 4.2 a | 104.0 ± 3.0 | 94.1 ± 2.0 a |
| Insulin (μg/dL) | 21.7 ± 10.5 | 7.5 ± 2.0 a | 9.6 ± 2.3 * | 7.7 ± 2.0 a |
| HOMA-IR (units) | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.9 ± 0.2 | 1.2 ± 0.3 |
| LEP (ng/dL) | 26.0 ± 4.2 | 18.7 ± 1.9 a | 42.4 ± 10.5 * | 29.8 ± 9.3 a |
| ADI (μg/dL) | 18.5 ± 1.5 | 21.1 ± 1.5 a | 19.2 ± 1.6 | 23.5 ± 2.4 a |
| Variable | WP | FP | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Kcal | 2146 ± 175 | 1889 ± 148 a | 2094 ± 194 | 1833 ± 158 a |
| Protein (g) | 92 ± 10 | 150 ± 14 a | 94 ± 7 | 140 ± 8 a |
| Protein (%) | 17 ± 2 | 33 ± 2 a | 20 ± 1 | 30 ± 2 a |
| Protein (g/kg) | 1.0 ± 0.12 | 1.7 ± 0.17 a | 0.9 ± 0.1 | 1.6 ± 0.2 a |
| Carbohydrates (g) | 253 ± 20 | 178 ± 19 a | 214 ± 17 | 158 ± 16 a |
| Carbohydrates (%) | 48 ± 2 | 38 ± 2 a | 43 ± 2 | 34 ± 2 a |
| Fat (g) | 77 ± 9 | 62 ± 4 | 75 ± 9 | 76 ± 12 |
| Fat (%) | 33 ± 2 | 30 ± 1 | 33 ± 2 | 36 ± 3 |
| Fiber (g) | 14 ± 1 | 26 ± 3 | 22 ± 3 | 23 ± 4 |
| Omega 3 | 2 ± 0.5 | 2 ± 1 | 1 ± 0.1 | 2 ± 0.5 |
| Hunger | 36 ± 8 | 31 ± 7 a | 46 ± 6 | 30 ± 8 a |
| Satiety | 23 ± 7 | 44 ± 5 a | 37 ± 6 | 44 ± 11 a |
| Desire to eat | 39 ± 10 | 38 ± 7 | 48 ± 6 | 31 ± 6 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arciero, P.J.; Edmonds, R.C.; Bunsawat, K.; Gentile, C.L.; Ketcham, C.; Darin, C.; Renna, M.; Zheng, Q.; Zhang, J.Z.; Ormsbee, M.J. Protein-Pacing from Food or Supplementation Improves Physical Performance in Overweight Men and Women: The PRISE 2 Study. Nutrients 2016, 8, 288. https://doi.org/10.3390/nu8050288
Arciero PJ, Edmonds RC, Bunsawat K, Gentile CL, Ketcham C, Darin C, Renna M, Zheng Q, Zhang JZ, Ormsbee MJ. Protein-Pacing from Food or Supplementation Improves Physical Performance in Overweight Men and Women: The PRISE 2 Study. Nutrients. 2016; 8(5):288. https://doi.org/10.3390/nu8050288
Chicago/Turabian StyleArciero, Paul J., Rohan C. Edmonds, Kanokwan Bunsawat, Christopher L. Gentile, Caitlin Ketcham, Christopher Darin, Mariale Renna, Qian Zheng, Jun Zhu Zhang, and Michael J. Ormsbee. 2016. "Protein-Pacing from Food or Supplementation Improves Physical Performance in Overweight Men and Women: The PRISE 2 Study" Nutrients 8, no. 5: 288. https://doi.org/10.3390/nu8050288