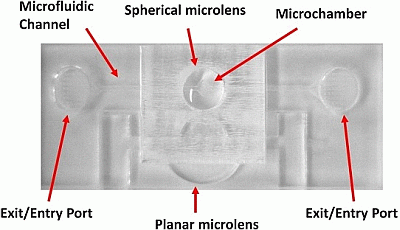
2.1. Materials and Fabrication
All microlens and microfluidic structures demonstrated in this work were manufactured using a Class 2 CO2 laser etching system (Helix 24, Epilog, Golden, CO, USA), which operates at a maximum power of 40 W, a maximum scan speed of 96 mm s−1, a wavelength of 10.6 μm with a spot size of 76–127 μm. All devices were manufactured from CLAREX® precision thin sheet PMMA, made by a specialist cell-casting technique, which ensures very high surface uniformity, and was purchased precast from Weatherall Equipment & Instruments Ltd (Bucks, UK). Designs of the plano-convex lenses were created using Corel Draw X4 (Corel Software, Ottawa, ON, Canada) interfaced directly with the CO2 laser.
Within the Epilog system, the CO
2 laser is mounted in the device, such that its focal point is directed into reflecting and focusing optical elements, which translate the beam in the
x- and
y-directions through the use of two stepper motors. Samples to be processed are brought into focus with the laser upon a working stage, which is translatable in the
z-direction. PMMA is processed based on its ability to absorb the radiation produced by the laser, with the power required to etch a given thickness depending on the melting and vaporisation temperatures of the polymer. The laser power and scan speed can be adjusted across a percentage scale in 1% increments, across either vector or raster power modes, as described in previous articles [
21,
22].
For the final manufacturing phase, the devices were constructed from a double-layer PMMA laminate, with the microfluidic channels and 2D lenses on the lower 1 mm-thick layer and plano-convex lenses on the upper layer. The upper layer also acts as a seal for the microfluidic channels and provides entry and exit ports for the test fluid, with flow being initiated and maintained through capillary action. Microchannels were fabricated in a single pass using a laser scan speed of 35% and power of 30%, with the laser set to low power raster mode, creating microchannels approximately 300 μm in depth. 2D lenses were created in a single pass of the laser with a scan speed of 30% and power of 80%, with the laser set to the high power vector mode to cut through the PMMA substrate, creating a 2D lens structure with a thickness equal to that of the substrate. The 2D lenses were singulated from the substrate to ease the polishing of the surfaces. They were also cut into a squared section of PMMA, such that they can be slotted back into the chip containing the channels for the subsequent bonding process. The polishing of the 2D lens surfaces is performed in an identical fashion to the plano-convex lenses, as described in
Section 2.2.
The devices were bonded using a thermal compressive bonding procedure, described in [
21]. The entire bonding procedure can be completed within approximately 2–3 h and leads to a permanent bonding of the PMMA through the controlled melting and re-solidification of the surfaces of the two PMMA layers. Briefly, the two laminate layers were covered with borosilicate glass microscope slides over the top and bottom layers and then placed between two 10 mm-thick steel plates and compressed together. The plates were then placed into a convection oven and heated under a slow thermal gradient (2–3 °C min
−1) above the glass transition temperature of the PMMA (170 °C), for a period of approximately 40 min. Following this, the oven is cooled to 80 °C and held for duration of 30 min before being cooled to room temperature.
2.2. Plano-Convex Lens Modelling and Fabrication
In addition to the unique manufacturing process that makes use of the graded power function of the laser engraving system, the 3D plano-convex lenses in this work yield several distinct advantages compared to the use of our previously demonstrated 2D lens structures. Firstly, the 2D lenses only allow for light to be directed within the plane of the chip, and the 3D lenses allow for light to be collected, or directed, orthogonal to the chip plane, making both lenses suitable for distinctly different applications. Therefore, collecting emission light orthogonal to the chip plane removes the cross-talk from excitation and emission light, thereby maximising the detection signal. For orthogonal chip light emission detection, the 2D lenses are not suitable. However, the 3D plano-convex lenses readily allow for this orientation, and their fabrication within the bulk chip substrate layer means that they can easily be integrated into the laminate chip bonding method used in this work. Additionally, the 3D lenses provide a greater collection efficiency of light, allowing for a near 2π collection angle, whereas collection with 2D lenses approximately has a collection angle of π. Ultimately, this makes the 2D lens structures ideal for excitation purposes, where light generally needs to be delivered in the chip plane, and the 3D plano-convex lenses are suited for emission collection optics.
In our previous work, we have demonstrated the CO
2 laser operating with either power on or off [
8,
21]. In this work, the laser was used in an analogue fashion to yield greater functionality during fabrication to create the 3D features. In that regard, a circular design, which comprises the plano-convex lens, was superimposed upon a rectangular area. The rectangular area is removed by direct etching using the laser system. The use of the rectangular area surrounding the lens is required to allow space for polishing of the lens surface post engraving. The power distribution of the laser was graded from full power at the rectangle area and circle edges and gradually moving to zero power at the centre of the circle. This resulted in the creation of a plano-convex lens in the centre of the rectangular etched area within the PMMA. The curvature of the lens can be adjusted depending upon the radius of the circle design and by control of the depth of etching in the low power, raster scan mode of the laser. Using the CO
2 laser at maximum power in raster scan mode,
Figure 1 illustrates the attained average lens depth of etch as a function of the laser scan speed for a 5-mm diameter lens fabricated into a 2 mm-thick PMMA substrate. Tests were also performed using multiple passes of the laser over a single target area, allowing for the maximum depth of etch to be extended beyond that of a single pass. During the subsequent passes, the laser spot is out of focus and imparts less power onto the target area. This has the effect of partially “cleaning” residual, condensed PMMA material. For this work, 2 passes were performed with the power set to maximum and with speeds of 45% and 60% for the first and second pass, respectively. This produced a lens structure with an approximate depth of 600 ± 30 μm, which was used as a standard depth to investigate various diameter lens structures.
Figure 1.
Ablation depth as a function of laser scan speed for maximum raster mode laser power.
Figure 1.
Ablation depth as a function of laser scan speed for maximum raster mode laser power.
Optical simulations were performed using OptiCAD 7.0 (OptiCAD Corporation, Santa Fe, NM, USA) modelling the focusing of light from a collimated white light source through a plano-convex spherical lens and onto a virtual detection area. This model mimics our experimental set-up allowing for the determination of the focal length of the lens. In all simulations, a refractive index for PMMA of 1.49 was used.
Figure 2 shows ray path simulations for a 5 mm-diameter plano-convex lens. Several lens diameters were examined, and a summary of the lens properties and predicted focal distances can be found in
Table 1. The simulations revealed the spherical aberrations that would be present in such a lens design, which results in a range over which the true focal distance of the lens will be found, and this range is expressed in
Table 1.
Figure 2.
(
a) Simulated results of the focusing of a collimated light source through a 5-mm plano-convex lens showing (
i) 3D and (
ii) a plan view of the lens and light rays; (
b) A diagram illustrating the parameters of the fabricated lenses within a PMMA bulk substrate, as stated in
Table 1.
Figure 2.
(
a) Simulated results of the focusing of a collimated light source through a 5-mm plano-convex lens showing (
i) 3D and (
ii) a plan view of the lens and light rays; (
b) A diagram illustrating the parameters of the fabricated lenses within a PMMA bulk substrate, as stated in
Table 1.
Table 1.
Theoretical results for various lens diameters and their respective focal lengths.
R1 is defined in
Figure 2.
Table 1.
Theoretical results for various lens diameters and their respective focal lengths. R1 is defined in Figure 2.
| Lens diameter (mm) | Lens height (mm) | R1 (mm) | Simulated focal range (mm) |
|---|
| 0.25 | 0.6 | 12.5 | 0.19–0.21 |
| 0.5 | 0.6 | 0.25 | 0.44–0.46 |
| 1 | 0.6 | 0.5 | 0.92–0.95 |
| 3 | 0.6 | 2.35 | 4.2–4.3 |
| 5 | 0.6 | 6 | 11.2–11.5 |
The plano-convex lenses were fabricated in a similar fashion to PMMA-based laser-ablated 2D lenses [
8]. The surfaces of the lenses were found to be rough, due to the nature of the thermal vaporisation and condensation process, as observed in
Figure 3a-i. Following the initial laser etching, the lenses were then polished manually by hand, by firstly sequentially sanding using 60, 240, 600, 1200, 2400 and 4000 grit wet and dry sandpaper to remove major defects. Lenses were sanded by placing a 1 cm
2 piece of wetted sand paper over the lens, applying light pressure with the index finger and rotating the paper in a clockwise circular manner, with the whole procedure taking approximately 5–6 min. This was followed by a two-stage hand polishing with an acrylic polymer cutting compound (Glass Polish Ltd., Tolworth, UK) to remove the majority of remaining abrasions and scratches, with this procedure taking approximately 5 min. Following polishing, the lenses underwent a final stage of thermal processing, whereby the substrate is heated above the glass transition temperature of the PMMA for 30 min, resulting in the liquefaction of the lens surface. At this stage, the surface tension effects during liquefaction remove any remaining minor defects, resulting in a high quality finish to the lens surface, as can be seen in
Figure 3a-ii,b. The process of defect removal had measured effects on the radius and height, and each was quantified by taking the average across 10 independently manufactured 5 mm-diameter lenses. The radiuses of the lenses were found to contain negligible differences, with an average reduction of 5–10 μm over the 5 mm of diameter. The height at the centre of the lens relative to the original substrate height was found to be reduced by approximately 250 ± 20 μm. These changes were factored into the optical simulations to reflect the true geometry of the manufactured lenses.
The graded spatial thermal processing of the PMMA substrate was examined over varying lens diameters to determine the limitations to the geometry of the manufactured lenses. The CO2 laser system has a minimum manufacturing resolution of approximately 100–150 μm. The smallest manufacturable size is likely to be significantly larger for the laser stepper motor to create the lens curvature upon the PMMA surface. Various-sized lenses were manufactured with diameters of 5 mm, 3 mm, 2 mm, 1 mm and 750 μm, and the quality of the final structures was assessed to determine the limitations of the proposed manufacturing process, as described later in the article. It was determined that the 5 mm-larger diameter lens structure was likely to be the most practical for implementation into a working device, given its focal length and the need to manually align the photodetector with the chip system. Therefore, work was focused primarily on the characterisation of this type of lens.
Figure 3.
(a) Photographs of a 5 mm, spherical PMMA lens (i) post-laser ablation and (ii) post-polishing and thermal annealing; (b) The same PMMA post-polished and thermally annealed lens in (i) side profile and (ii) overhead profile.
Figure 3.
(a) Photographs of a 5 mm, spherical PMMA lens (i) post-laser ablation and (ii) post-polishing and thermal annealing; (b) The same PMMA post-polished and thermally annealed lens in (i) side profile and (ii) overhead profile.
2.3. Fluorescence and Light Detection
The manufactured lenses were validated through the detection of both light from various LED sources and from the emission of excited fluorophores placed into microfluidic systems. For all characterisation-based experiments, detection was achieved using a USB-based spectrometer (USB2000+, Ocean Optics, Dunedin, FL, USA) in conjunction with a 200-μm coupling optical fibre (Numerical Aperture (NA) = 0.22) mounted upon an
x-y-z translational stage. Focal length determinations were achieved using a blue collimated LED source (M470L3-C1, Thorlabs, Ely, UK) and the diameter of the light column controlled by an in-house build optical aperture. A schematic of the experimental setup can be seen in
Figure 4a.
For testing in the microfluidic system, serial dilutions of fluorescein isothiocyanate (FITC) dye (λ
Excitation = 494 nm and λ
Emission = 518 nm) were used as a demonstrator for fluorescence detection. In this work, a 1-nM FITC solution was used for all testing of lens properties. This was made fresh in a stock solution combined on the first instance with ethanol, following the manufacturer’s recommendations, and then subsequently diluted with deionised water. As ethanol degrades PMMA, which was used as the vessel for FITC, a water-based dilution methodology was used to preserve the integrity of the microfluidic channels. Excitation of the fluorophores was achieved using a 3-mm Nichia ultra-bright blue LED (NSPB300A, RS Components, Glasgow, UK) and a band pass excitation filter (FB490-10, Thorlabs, UK) with λ
Trans = 490 ± 5 nm (approximately 48% transmission), both mounted into a custom-built holder cradle. Fluorescence detection measurement was achieved using a H9858 photosensor module (Hamamatsu, Welwyn Garden City, UK), in conjunction with an FITC-optimised band pass emission filter (HQ535-50m, Chroma, Bellows Falls, VT, USA), λ
Trans = 535 ± 25 nm (approximately 75%–80% transmission). A schematic of the experimental set up can be seen in
Figure 4b. The photodetector and emission filter were both housed within a custom-built cradle, to preserve the alignment of the two components and to reduce background light from entering the active area of the detector. A secondary rig was built to house the excitation LED/emission filter and to create a cradle into which the chip could be placed, such that its final orientation would leave the 2D planar lens and microchamber of the fluidic chip in direct alignment with the LED and photodetector, respectively. The photomultiplier module, LED and filters were set up in a configuration as illustrated in
Figure 5b, and the photodetector was mounted a distance of 40 mm above the bottom of the chip holding cradle. As a benchmark comparison, fluorescence measurements were performed using a 10× microscope objective (Thorlabs, UK) as the light collection optics, placed approximately 500 μm above the fluidic microchamber, in a configuration described previously [
8]. Such a system has been demonstrated previously by our group to provide FITC detection limits of 10 fM, which is comparable to the detection capabilities of commercial fluorescence reader systems. The use of this benchmark setup allows us to examine the efficiency of the optical component only, as we shall keep the excitation and detection apparatus the same. This therefore eliminates the variability from factors, such as detector sensitivity and excitation power emission, that have a different fluorescence setup being used, thereby providing a more fair comparison of the devised plano-convex lenses against commercial-grade optics.
Figure 4.
Schematic of the experimental setup to determine (a) the lens focal length and (b) the FITC fluorescence intensity measurements.
Figure 4.
Schematic of the experimental setup to determine (a) the lens focal length and (b) the FITC fluorescence intensity measurements.