Beeswax-Colophony Blend: A Novel Green Organic Coating for Protection of Steel Drinking Water Storage Tanks
Abstract
:1. Introduction

2. Materials and Methods
2.1. Thermodynamic Calculations
2.2. Coating Preparation
2.3. Corrosion Measurements
2.3.1. Sample Preparation
2.3.2. Experimental Procedure
2.4. Preliminary Microbiological Evaluations
2.4.1. Simulated Water Tank


2.4.2. Experimental Procedure
Sampling
| Parameter | Conductivity (μS/cm) | pH | [Ca2+] (mg L−1) | [Mg2+] (mg L−1) | [Na+] (mg L−1) | [K+] (mg L−1) | [Cl−] (mg L−1) | [NO3−] (mg L−1) | [SO42−] (mg L−1) | [HCO3−] (mg L−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Value | 1350 | 7.10 | 119 | 46 | 101 | 1.20 | 156 | 101 | 147 | 3.20 |
HPC Procedure
Micro-Pollutants Measurements
2.5. Surface Characterization
3. Results and Discussion
3.1. Thermodynamic Calculations


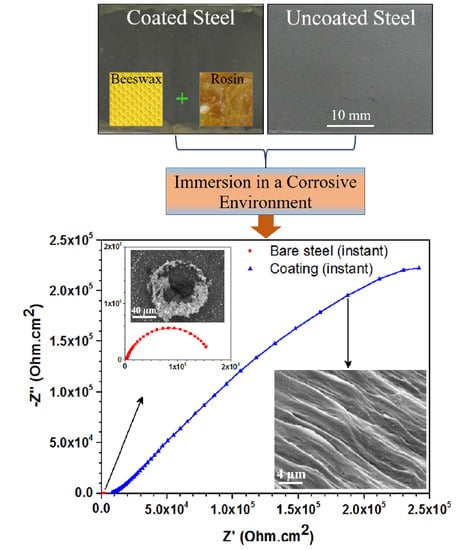
3.2. Electrochemical Measurements

- ω = radial frequency (rad s−1)
- DO = diffusion coefficient of the oxidant (cm2 s−1)
- DR = diffusion coefficient of the reductant (cm2 s−1)
- A = surface area of the electrode (cm2)
- n = number of electrons involved
- F = Faradays constant
- R = gas constant
- T = temperature (K)
- C*o = concentration of oxidant in the bulk
- C*R = concentration of reductant in the bulk
- J = (−1)0.5
- ZO = finite Warburg impedance
- δ = Nernst diffusion layer thickness (cm)
- D = some average value of the diffusion coefficients of the diffusing species (cm2 s−1)

| Time (h) | Rf (Ohm cm²) | Rct (Ohm cm2) | nf | ndl | pf (F cm−2) | pdl (F cm−2) | Wor (Ohm s−0.5) | Woc (s0.5) | W (Ohm s−0.5) |
|---|---|---|---|---|---|---|---|---|---|
| Bare steel (instant) | --- | 1.63 × 103 | --- | 0.78 | --- | 6.3 × 10−4 | --- | --- | --- |
| Coating (Instant) | 6.67 × 103 | 8.11 × 105 | 0.28 | 0.73 | 3.73 × 10−5 | 1.81 × 10−5 | --- | --- | --- |
| 7 | 6.07 × 103 | 1.50 × 106 | 0.22 | 0.71 | 5.57 × 10−5 | 1.49 × 10−5 | --- | --- | --- |
| 22 | 6.44 × 103 | 1.60 × 106 | 0.21 | 0.70 | 5.32 × 10−5 | 1.52 × 10−5 | --- | --- | --- |
| 46 | --- | 9.10 × 103 | 0.50 | 0.78 | 8.40 × 10−6 | 1.25 × 10−9 | 1.56 × 105 | 0.95 | --- |
| 70 | --- | 7.38 × 103 | 0.53 | 0.79 | 1.17 × 10−5 | 1.10 × 10−9 | 2.04 × 105 | 1.01 | --- |
| 94 | --- | 7.43 × 103 | 0.52 | 0.78 | 1.12 × 10−5 | 1.29 × 10−9 | 1.62 × 105 | 1.02 | --- |
| 118 | --- | 8.03 × 103 | 0.48 | 0.78 | 1.05 × 10−5 | 1.32 × 10−9 | 1.35 × 105 | 1.07 | --- |
| 142 | --- | 8.57 × 103 | 0.52 | 0.78 | 1.10 × 10−5 | 1.55 × 10−9 | 1.82 × 105 | 1.15 | --- |
| 166 | --- | 8.27 × 103 | 0.52 | 0.78 | 1.09 × 10−5 | 1.87 × 10−11 | 1.93 × 105 | 1.34 | --- |
| 214 | --- | 2.26 × 105 | 0.44 | 0.93 | 1.83 × 10−6 | 2.90 × 10−11 | 6.21 × 105 | 3.22 | --- |
| 262 | --- | 2.17 × 105 | 0.45 | 0.91 | 1.78 × 10−6 | 3.66 × 10−11 | 5.92 × 105 | 3.42 | --- |
| 310 | --- | 2.50 × 105 | 0.43 | 0.92 | 1.50 × 10−6 | 3.54 × 10−11 | 7.03 × 105 | 3.52 | --- |
| 358 | --- | 2.60 × 105 | 0.42 | 0.90 | 1.39 × 10−6 | 4.21 × 10−11 | 6.61 × 105 | 3.64 | --- |
| 406 | --- | 2.64 × 105 | 0.38 | 0.91 | 1.24 × 10−6 | 3.59 × 10−11 | 5.90 × 105 | 3.65 | --- |
| 454 | --- | 2.64 × 105 | 0.38 | 0.92 | 1.24 × 10−6 | 3.59 × 10−11 | 5.89 × 105 | 3.65 | --- |
| 622 | 5.67 × 105 | 4.13 × 105 | 0.58 | 0.98 | 3.59 × 10−7 | 1.33 × 10−11 | --- | --- | 1.03 × 106 |
| 790 | 4.53 × 105 | 4.53 × 105 | 0.59 | 0.97 | 3.02 × 10−7 | 1.49 × 10−11 | --- | --- | 1.11 × 106 |
| 958 | 4.79 × 105 | 4.96 × 105 | 0.60 | 0.92 | 3.92 × 10−7 | 2.54 × 10−11 | --- | --- | 1.37 × 106 |
| 1126 | 2.74 × 105 | 5.68 × 105 | 0.62 | 0.83 | 2.35 × 10−7 | 6.09 × 10−11 | --- | --- | 1.30 × 106 |
| 1294 | 2.65 × 105 | 5.55 × 105 | 0.63 | 0.83 | 2.49 × 10−7 | 5.67 × 10−11 | --- | --- | 1.19 × 106 |
| 1462 | 2.50 × 105 | 5.84 × 105 | 0.62 | 0.86 | 2.50 × 10−7 | 4.57 × 10−11 | --- | --- | 1.19 × 106 |

3.3. Preliminary Chemical and Microbiological Evaluations
3.3.1. HPC Procedure
3.3.2. Micro-Pollutants Measurements
| Sample | Detected Compounds (ppb) | |||
|---|---|---|---|---|
| Toluene | Chloroform | Bromodichloromethane | Dibromochloromethane | |
| 1 | 0.83 | 0.73 | 5.81 | 10.48 |
| 2 | 1.2 | Not detected | Not detected | 17.7 |
3.4. Surface Characterization


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morton, L.H.G.; Surman, S.B. Biofilms in biodeterioration—A review. Int. Biodeterior. Biodegrad. 1994, 34, 203–221. [Google Scholar] [CrossRef]
- Volk, C.; Dundore, E.; Schiermann, J.; le Chevallier, M. Practical evaluation of iron corrosion control in a drinking water distribution system. Water Res. 2000, 34, 1967–1974. [Google Scholar] [CrossRef]
- Videla, H.A. Prevention and control of biocorrosion. Int. Biodeterior. Biodegrad. 2002, 49, 259–270. [Google Scholar] [CrossRef]
- Teng, F.; Guan, Y.T.; Zhu, W.P. Effect of biofilm on cast iron pipe corrosion in drinking water distribution system: Corrosion scales characterization and microbial community structure investigation. Corros. Sci. 2008, 50, 2816–2823. [Google Scholar] [CrossRef]
- Świetlik, J.; Raczyk-Stanisławiak, U.; Piszora, P.; Nawrocki, J. Corrosion in drinking water pipes: The importance of green rusts. Water Res. 2012, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Zhang, Y.; Hu, C.; Yang, M. Characterization of the bacterial communities and iron corrosion scales in drinking groundwater distribution systems with chlorine/chloramine. Int. Biodeterior. Biodegrad. 2014, 96, 71–79. [Google Scholar] [CrossRef]
- Usher, K.M.; Kaksonen, A.H.; Cole, I.; Marney, D. Critical review: Microbially influenced corrosion of buried carbon steel pipes. Int. Biodeterior. Biodegrad. 2014, 93, 84–106. [Google Scholar] [CrossRef]
- Wagner, P.A.; Little, B.J.; Hart, K.R.; Ray, R.I. Biodegradation of composite materials. Int. Biodeterior. Biodegrad. 1996, 38, 125–132. [Google Scholar] [CrossRef]
- Beech, I.B. Corrosion of technical materials in the presence of biofilms—Current understanding and state-of-the art methods of study. Int. Biodeterior. Biodegrad. 2004, 53, 177–183. [Google Scholar] [CrossRef]
- El-Shamy, A.M.; Soror, T.Y.; El-Dahan, H.A.; Ghazy, E.A.; Eweas, A.F. Microbial corrosion inhibition of mild steel in salty water environment. Mater. Chem. Phys. 2009, 114, 156–159. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthkrishnan, S.; Venkatachari, G. Corrosion protection of steel by polyaniline blended coating. Electrochim. Acta 2006, 51, 6313–6319. [Google Scholar] [CrossRef]
- Kim, H.J. Development of a new organic composite coated steel sheet with high corrosion resistance. Surf. Coat. Technol. 1994, 70, 37–41. [Google Scholar] [CrossRef]
- Gonçalves, G.S.; Baldissera, A.F.; Rodrigues, L.F., Jr.; Martini, E.M.A.; Ferreira, C.A. Alkyd coatings containing polyanilines for corrosion protection of mild steel. Synth. Met. 2011, 161, 313–323. [Google Scholar] [CrossRef]
- Gaillard, Y.; Girard, M.; Monge, G.; Burr, A.; Ceretti, E.D.; Felder, E. Superplastic behavior of rosin/beeswax blends at room temperature. J. Appl. Polym. Sci. 2013, 128, 2713–2719. [Google Scholar] [CrossRef]
- Kameda, T. Molecular structure of crude beeswax studied by solid-state 13C NMR. J. Insect Sci. 2004. [Google Scholar] [CrossRef]
- Kameda, T.; Tamada, Y. Variable-temperature 13C solid-state NMR study of the molecular structure of honeybee wax and silk. Int. J. Biol. Macromol. 2009, 44, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Naderi, R.; Attar, M.M.; Moayed, M.H. EIS examination of mill scale on mild steel with polyester-epoxy powder coating. Prog. Org. Coat. 2004, 50, 162–165. [Google Scholar] [CrossRef]
- Luo, W.; Li, T.; Wang, C.; Huang, F. Discovery of Beeswax as binding agent on a 6th-century BC Chinese Turquoise-inlaid Bronze sword. J. Archaeol. Sci. 2012, 39, 1227–1237. [Google Scholar] [CrossRef]
- Scholz, G.; Militz, H.; Gascón-Garrido, P.; Ibiza-Palacios, M.S.; Oliver-Villanueva, J.V.; Peters, B.C.; Fitzgerald, C.J. Improved termite resistance of wood by wax impregnation. Int. Biodeterior. Biodegrad. 2010, 64, 688–693. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Gandini, A. Chapter 4—Rosin: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, N.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 67–88. [Google Scholar]
- Mellema, M. Co-crystals of Beeswax and Various Vegetable Waxes with Sterols Studied by X-ray Diffraction and Differential Scanning Calorimetry. J. Am. Oil Chem. Soc. 2009, 86, 499–505. [Google Scholar] [CrossRef]
- Gaillard, Y.; Mija, A.; Burr, A.; Darque-Ceretti, E.; Felder, E.; Sbirrazzuoli, N. Green material composites from renewable resources: Polymorphic transitions and phase diagram of beeswax/rosin resin. Thermochim. Acta 2011, 521, 90–97. [Google Scholar] [CrossRef]
- Yasuda, H.; Yu, Q.S.; Chen, M. Interfacial factors in corrosion protection: An EIS study of model systems. Prog. Org. Coat. 2001, 41, 273–279. [Google Scholar] [CrossRef]
- Bierwagen, G.; Tallman, D.; Li, J.; He, L.; Jeffcoate, C. EIS studies of coated metals in accelerated exposure. Prog. Org. Coat. 2003, 46, 149–158. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of water transport behavior and impedance models of epoxy-coated metals in NaCl solution by EIS. Prog. Org. Coat. 2004, 51, 145–151. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S. An EIS study of ion diffusion through organic coatings. Electrochim. Acta 2006, 51, 1736–1744. [Google Scholar] [CrossRef]
- Hinderliter, B.R.; Croll, S.G.; Tallman, D.E.; Su, Q.; Bierwagen, G.P. Interpretation of EIS data from accelerated exposure of coated metals based on modeling of coating physical properties. Electrochim. Acta 2006, 51, 4505–4515. [Google Scholar] [CrossRef]
- Zuo, Y.; Pang, R.; Li, W.; Xiong, J.P.; Tang, Y.M. The evaluation of coating performance by the variations of phase angles in middle and high frequency domains of EIS. Corros. Sci. 2008, 50, 3322–3328. [Google Scholar] [CrossRef]
- Ito, D.; Yokoyama, T.; Okazaki, S. Evaluation of Blister Growth of High Performance Organic Coatings by Electrochemical Impedance Measurements. ECS Trans. 2012, 41, 55–60. [Google Scholar]
- Kong, D.; Wang, Y.; Zhang, W.; Wang, W.; Liu, X.; Wang, J. Correlation between electrochemical impedance and current distribution of carbon steel under organic coating. Mater. Corros. 2012, 63, 475–480. [Google Scholar] [CrossRef]
- Mills, D.; Jamali, S.; Tobiszewski, M.T. Developing electrochemical measurements in order to assess anti-corrosive coatings more effectively. Prog. Org. Coat. 2012, 74, 385–390. [Google Scholar] [CrossRef]
- Amand, S.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Constant-phase-element behavior caused by inhomogeneous water uptake in anti-corrosion coatings. Electrochim. Acta 2013, 87, 693–700. [Google Scholar] [CrossRef]
- Upadhyay, V.; Harkal, U.; Webster, D.; Bierwagen, G. Preliminary investigation of the impact of polymer composition on electrochemical properties of coatings as determined by electrochemical impedance spectroscopy. J. Coat. Technol. Res. 2013, 10, 865–878. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y. Comparisons of clear coating degradation in NaCl solution and pure water. Prog. Org. Coat. 2013, 76, 1674–1682. [Google Scholar] [CrossRef]
- Tölgyessy, P.; Vrana, B.; Krascsenits, Z. Development of a screening method for the analysis of organic pollutants in water using dual stir bar sorptive extraction-thermal desorption-gas chromatography-mass spectrometry. Talanta 2011, 87, 152–160. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Heterotrophic Plate Counts and Drinking-Water Safety; Bartram, J., Cotruvo, J., Exner, M., Fricker, C., Glasmacher, A., Eds.; IWA Publishing: Padstow, UK, 2003. [Google Scholar]
- Medusa Thermodynamic Software; KTH Royal Institute of Technology: Stockholm, Sweden, 2009.
- Poelman, M.; Recloux, I.; Cornil, N.; Blandin, N.; Deronne, L.; LeDisert, Y.; Olivier, M.-G. Electrochemical study of the corrosion behaviour at the edges of electrocoated steel. Prog. Org. Coat. 2012, 74, 453–460. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G.A. Inverse Problem in Potentiodynamic Electrochemical Impedance. Available online: http://www.abc.chemistry.bsu.by/vi/analyser/ (accessed on 14 September 2015).
- Allen, M.J.; Edberg, S.C.; Reasoner, D.J. Heterotrophic plate count bacteria—What is their significance in drinking water? Int. J. Food Microbiol. 2004, 92, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Caruso, M.; Trovatelli, F.; Tosques, M. Comparison of plate count agar and R2A medium for enumeration of heterotrophic bacteria in natural mineral water. World J. Microbiol. Biotechnol. 1998, 14, 727–730. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Recommendations; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Chambers, L.D.; Wharton, J.A.; Wood, R.J.K.; Walsh, F.C.; Stokes, K.R. Techniques for the measurement of natural product incorporation into an antifouling coating. Prog. Org. Coat. 2014, 77, 473–484. [Google Scholar] [CrossRef]
- Mansfeld, F.; Little, B. A technical review of electrochemical techniques applied to microbiologically influenced corrosion. Corros. Sci. 1991, 32, 247–272. [Google Scholar] [CrossRef]
- George, R.P.; Marshall, D.; Newman, R.C. Mechanism of a MIC probe. Corros. Sci. 2003, 45, 1999–2015. [Google Scholar] [CrossRef]
- Standardization ECf. Water Quality-Sampling for Microbiological Analysis; British Standards Institute: Brussels, Belgium, 2006. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdikheibari, S.; Parvizi, R.; Moayed, M.H.; Zebarjad, S.M.; Sajjadi, S.A. Beeswax-Colophony Blend: A Novel Green Organic Coating for Protection of Steel Drinking Water Storage Tanks. Metals 2015, 5, 1645-1664. https://doi.org/10.3390/met5031645
Abdikheibari S, Parvizi R, Moayed MH, Zebarjad SM, Sajjadi SA. Beeswax-Colophony Blend: A Novel Green Organic Coating for Protection of Steel Drinking Water Storage Tanks. Metals. 2015; 5(3):1645-1664. https://doi.org/10.3390/met5031645
Chicago/Turabian StyleAbdikheibari, Sara, Reza Parvizi, Mohammad Hadi Moayed, Seyed Mojtaba Zebarjad, and Seyed Abdolkarim Sajjadi. 2015. "Beeswax-Colophony Blend: A Novel Green Organic Coating for Protection of Steel Drinking Water Storage Tanks" Metals 5, no. 3: 1645-1664. https://doi.org/10.3390/met5031645