Amino Functionalized Micro-Mesoporous Hybrid Particles for the Sustained Release of the Antiretroviral Drug Tenofovir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Functionalization of the Porous Hybrid Particles. Characterization Methods
2.2. Cytotoxicity Evaluation
2.3. Drug Loading and Release Experiments
3. Results
3.1. Characterization of the Functionalized Porous Hybrid Particles
3.2. Toxicity Evaluation
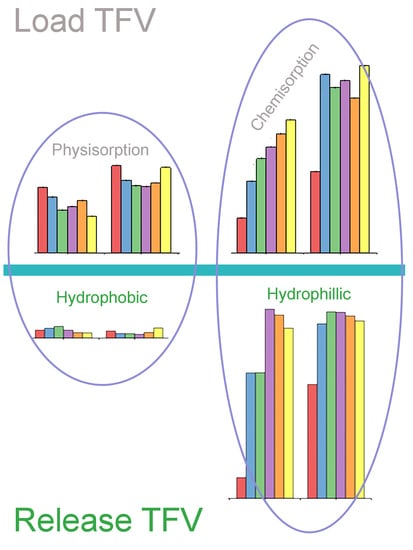
3.3. Drug Loading Capacity
3.4. Drug Releasing Capability and Pharmacokinetical Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sher, P.; Ingavle, G.; Ponrathnam, S.; Pawar, A.P. Low density porous carrier: Drug adsorption and release study by response surface methodology using different solvents. Int. J. Pharm. 2007, 331, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Paradkar, A. Porous polystyrene beads as carriers for self-emulsifying system containing loratadine. Aaps Pharmscitech 2006, 7, E199–E205. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Tokumitsu, K.; Matsuda, Y. Solid dosage form preparations from oily medicines and their drug release. Effect Of degree of surface-modification of silica gel on the drug release from phytonadione-loaded silica gels. J. Control. Release 2000, 67, 369–384. [Google Scholar] [CrossRef]
- Shen, S.; Chow, P.S.; Chen, F.; Tan, R.B. Submicron particles of SBA-15 modified with MgO as carriers for controlled drug delivery. Chem. Pharm. Bull. 2007, 55, 985–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doadrio, A.L.; Salinas, A.J.; Sanchez-Montero, J.M.; Vallet-Regi, M. Drug release from ordered mesoporous silicas. Curr. Pharm. Des. 2015, 21, 6213–6819. [Google Scholar] [CrossRef] [Green Version]
- Szegedi, A.; Popova, M.; Goshev, I.; Mihály, J. Effect of amine functionalization of spherical MCM-41 and SBA-15 on controlled drug release. J. Solid State Chem. 2011, 184, 1201–1207. [Google Scholar] [CrossRef]
- Ito, Y.; Kusawake, T.; Prasad, Y.V.; Sugioka, N.; Shibata, N.; Takada, K. Preparation and evaluation of oral solid heparin using emulsifier and adsorbent for in vitro and in vivo studies. Int. J. Pharm. 2006, 317, 114–119. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Larionova, N.I.; Sukhorukov, G.B. Protein encapsulation via porous CaCO3 microparticles templating. Biomacromolecules 2004, 5, 1962–1972. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jiang, H.; Wan, L.; Zhao, Q.; Jiang, T.; Wang, B.; Wang, S. Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomater. 2015, 13, 354–363. [Google Scholar] [CrossRef]
- Ahuja, G.; Pathak, K. Porous carriers for controlled/modulated drug delivery. Indian J. Pharm. Sci. 2009, 71, 599–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamayo, A.; Ruiz-Caro, R.; Mazo, A.; Veiga-Ochoa, M.; Rubio, J. Chemical oxidation of silicon oxycarbide ceramics for advanced drug delivery systems. J. Mater. Sci. 2016, 51, 1382–1391. [Google Scholar] [CrossRef]
- Tamayo, A.; Tellez, L.; Rodriguez-Reyes, M.; Mazo, M.A.; Rubio, F.; Rubio, J. Surface properties of bioactive TEOS-PDMS-TiO2-CaO ormosils. J. Mater. Sci. 2014, 49, 4656–4669. [Google Scholar] [CrossRef]
- Chen, Q.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and mechanical properties of PDMS-modified CaO–SiO2–TiO2 hybrids prepared by sol-gel process. J. Biomed. Mater. Res. 2000, 51, 605–611. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Kawashita, M.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and mechanical properties of polydimethylsiloxane (PDMS)-CaO-SiO2 hybrids with different PDMS contents. J. Sol Gel Sci. Technol. 2001, 21, 75–81. [Google Scholar] [CrossRef]
- MacCallum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B.; et al. Liquid-infused silicone as a biofouling-free medical material. ACS Biomater. Sci. Eng. 2015, 1, 43–51. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, J.-H.; Kim, J.H.; Jeong, S.M.; Lee, S.-H. Flexible, stretchable and implantable PDMS encapsulated cable for implantable medical device. Biomed. Eng. Lett. 2011, 1, 199. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft contact lens polymers: An evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.K.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- McConville, C.; Boyd, P.; Major, I. Efficacy of tenofovir 1% vaginal gel in reducing the risk of HIV-1 and HSV-2 infection. Clin. Med. Insights Women Health 2014, 7, CMWH–S10353. [Google Scholar] [CrossRef] [Green Version]
- Veiga-Ochoa, M.-D.; Ruiz-Caro, R.; Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F. Vaginal formulations for prevention of sexual transmission of HIV. In Advances in HIV and AIDS Control; IntechOpen: London, UK, 2018; p. 227. [Google Scholar]
- Martin-Illana, A.; Cazorla-Luna, R.; Notario-Perez, F.; Bedoya, L.M.; Ruiz-Caro, R.; Veiga, M.D. Freeze-dried bioadhesive vaginal bigels for controlled release of Tenofovir. Eur. J. Pharm. Sci. 2019, 127, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Notario-Perez, F.; Ruiz-Caro, R.; Veiga-Ochoa, M.-D. Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: From past failures to future hopes. Drug Des. Dev. Ther. 2017, 11, 1767–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazorla-Luna, R.; Martin-Illana, A.; Notario-Perez, F.; Miguel Bedoya, L.; Tamayo, A.; Ruiz-Caro, R.; Rubio, J.; Veiga, M.-D. Vaginal polyelectrolyte layer-by-layer films based on chitosan derivatives and eudragit S100 for pH responsive release of tenofovir. Mar. Drugs 2020, 18, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazorla-Luna, R.; Notario-Perez, F.; Martin-Illana, A.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Ruiz-Caro, R.; Veiga, M.-D. Development and In Vitro/Ex Vivo characterization of vaginal mucoadhesive bilayer films based on ethylcellulose and biopolymers for vaginal sustained release of tenofovir. Biomacromolecules 2020, 21, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Notario-Perez, F.; Martin-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Mucoadhesive vaginal discs based on cyclodextrin and surfactants for the controlled release of antiretroviral drugs to prevent the sexual transmission of HIV. Pharmaceutics 2020, 12, 321. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Krug, H.F. Quality Handbook—Standard Procedures for Nanoparticle Testing; Nanomune: Gipuzkoa, Switzerland, 2011. [Google Scholar]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Marques, M.R.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Almeida, R.M.; Guiton, T.A.; Pantano, C.G. Detection of LO mode in v-SiO2 by infrared diffuse reflectance spectroscopy. J. Non Cryst. Solids 1990, 119, 238–241. [Google Scholar] [CrossRef]
- Chmel, A.; Mazurina, E.K.; Shashkin, V.S. Vibrational spectra and deffect structure of silica prepared by non-organic sol-gel process. J. Non Cryst. Solids 1990, 122, 285–290. [Google Scholar] [CrossRef]
- Babonneau, F.; Thorne, K.; Mackenzie, J.D. Dimethyldiethoxysilane/tetraethoxysilane copolymers: Precursors for the silicon-carbon-oxygen system. Chem. Mater. 1989, 1, 554–558. [Google Scholar] [CrossRef]
- Palencia, C.; Rubio, J.; Rubio, F.; Fierro, J.L.G.; Luis Oteo, J. Silane coupling agent structures on carbon nanofibers. J. Nanosci. Nanotechnol. 2011, 11, 4142–4152. [Google Scholar] [CrossRef] [PubMed]
- Culler, S.R.; Ishida, H.; Koenig, J.L. FT-IR characterization of the reaction at the silane/matrix resin interphase of composite materials. J. Colloid Interface Sci. 1986, 109, 1–10. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Zidan, A.S.; Spinks, C.; Fortunak, J.; Habib, M.; Khan, M.A. Near-infrared investigations of novel anti-HIV tenofovir liposomes. Aaps J. 2010, 12, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Ramkumaar, G.; Srinivasan, S.; Bhoopathy, T.; Gunasekaran, S. Vibrational spectroscopic studies of tenofovir using density functional theory method. J. Chem. 2012. [Google Scholar] [CrossRef]
- Tamayo, A.; Mazo, M.A.; Veiga, M.D.; Ruiz-Caro, R.; Notario-Pérez, F.; Rubio, J. Drug kinetics release from Eudragit—Tenofovir@SiOC tablets. Mater. Sci. Eng. C 2017, 75, 1097–1105. [Google Scholar] [CrossRef]
- Shurshina, A.; Galina, A.; Kulish, E. Kinetics of the release of a drug from a soluble or hydrolyzable polymer matrix. Russ. J. Phys. Chem. B 2016, 10, 1001–1006. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Song, S.-W.; Hidajat, K.; Kawi, S. Functionalized SBA-15 materials as carriers for controlled drug delivery: Influence of surface properties on matrix—Drug interactions. Langmuir 2005, 21, 9568–9575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dunphy, D.R.; Jiang, X.; Meng, H.; Sun, B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.; Ji, Z.; et al. Processing pathway dependence of amorphous silica nanoparticle toxicity: Colloidal vs Pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavan, C.; Delle Piane, M.; Gullo, M.; Filippi, F.; Fubini, B.; Hoet, P.; Horwell, C.J.; Huaux, F.; Lison, D.; Giudice, C.L. The puzzling issue of silica toxicity: Are silanols bridging the gaps between surface states and pathogenicity? Part. Fibre Toxicol. 2019, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Dierker, C.; Rommel, C.; Hahn, D.; Wohlleben, W.; Schulze-Isfort, C.; Göbbert, C.; Voetz, M.; Hardinghaus, F.; Schnekenburger, J. Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays. Part. Fibre Toxicol. 2011, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-S.; Chang, K.L.B.; Hwang, D.-F.; Kong, Z.-L. In Vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ. Sci. Technol. 2007, 41, 2064–2068. [Google Scholar] [CrossRef]
- Aissaoui, N.; Bergaoui, L.; Landoulsi, J.; Lambert, J.-F.; Boujday, S. Silane layers on silicon surfaces: Mechanism of interaction, stability, and influence on protein adsorption. Langmuir 2012, 28, 656–665. [Google Scholar] [CrossRef]
- Reich, S.-J.; Svidrytski, A.; Höltzel, A.; Florek, J.; Kleitz, F.; Wang, W.; Kübel, C.; Hlushkou, D.; Tallarek, U. Hindered diffusion in ordered mesoporous silicas: Insights from pore-scale simulations in physical reconstructions of SBA-15 and KIT-6 silica. J. Phys. Chem. C 2018, 122, 12350–12361. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, L.; Monduzzi, M.; Salis, A. Adsorption and release of ampicillin antibiotic from ordered mesoporous silica. J. Colloid Interface Sci. 2017, 497, 217–225. [Google Scholar] [CrossRef]
- Kjellman, T.; Xia, X.; Alfredsson, V.; Garcia-Bennett, A.E. Influence of microporosity in SBA-15 on the release properties of anticancer drug dasatinib. J. Mater. Chem. B 2014, 2, 5265–5271. [Google Scholar] [CrossRef]
- Dolinina, E.S.; Parfenyuk, E.V. Kinetics and mechanism of the adsorption of the drug molsidomine on phenyl-modified mesoporous silica materials at different pH. Russ. J. Phys. Chem. A 2015, 89, 1293–1299. [Google Scholar] [CrossRef]
- Karim, A.H.; Jalil, A.A.; Triwahyono, S.; Sidik, S.M.; Kamarudin, N.H.N.; Jusoh, R.; Jusoh, N.W.C.; Hameed, B.H. Amino modified mesostructured silica nanoparticles for efficient adsorption of methylene blue. J. Colloid Interface Sci. 2012, 386, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, N.H.N.; Jalil, A.A.; Triwahyono, S.; Salleh, N.F.M.; Karim, A.H.; Mukti, R.R.; Hameed, B.H.; Ahmad, A. Role of 3-aminopropyltriethoxysilane in the preparation of mesoporous silica nanoparticles for ibuprofen delivery: Effect on physicochemical properties. Microporous Mesoporous Mater. 2013, 180, 235–241. [Google Scholar] [CrossRef]
- Kamarudin, N.H.N.; Jalil, A.A.; Triwahyono, S.; Artika, V.; Salleh, N.F.M.; Karim, A.H.; Jaafar, N.F.; Sazegar, M.R.; Mukti, R.R.; Hameed, B.H.; et al. Variation of the crystal growth of mesoporous silica nanoparticles and the evaluation to ibuprofen loading and release. J. Colloid Interface Sci. 2013, 421, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.B.; Jolly, M.R.; Kumbhar, D.D. Engineering of a hybrid polymer–lipid nanocarrier for the nasal delivery of tenofovir disoproxil fumarate: Physicochemical, molecular, microstructural, and stability evaluation. Eur. J. Pharm. Sci. 2015, 71, 99–111. [Google Scholar] [CrossRef]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Jesus, M.; Grazu, V. Nanobiotechnology: Inorganic Nanoparticles vs. Organic Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4. [Google Scholar]
- Chen, N.-T.; Cheng, S.-H.; Souris, J.S.; Chen, C.-T.; Mou, C.-Y.; Lo, L.-W. Theranostic applications of mesoporous silica nanoparticles and their organic/inorganic hybrids. J. Mater. Chem. B 2013, 1, 3128–3135. [Google Scholar] [CrossRef]
- Belot, V.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Thermal reactions occurring during pyrolysis of cross-linked polysiloxane gels, precursors to silicon oxycarbide glasses. J. Non Cryst. Solids 1992, 147, 52–55. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]










| % APS | 500 | 600 | 700 | 800 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BET | Vmic | Vmeso | BET | Vmic | Vmeso | BET | Vmic | Vmeso | BET | Vmicr | Vmeso | |
| (m2/g) | cm3/g | (m2/g) | cm3/g | (m2/g) | cm3/g | (m2/g) | cm3/g | |||||
| 0 | 553 | 0.178 | 0.908 | 710 | 0.260 | 1.176 | 611 | 0.208 | 1.063 | 658 | 0.180 | 1.242 |
| 0.3 | 500 | 0.146 | 0.910 | 704 | 0.252 | 1.253 | 571 | 0.164 | 1.000 | 530 | 0.250 | 0.851 |
| 0.5 | 484 | 0.164 | 0.872 | 702 | 0.202 | 1.220 | 570 | 0.189 | 0.992 | 518 | 0.195 | 0.853 |
| 1 | 480 | 0.131 | 0.861 | 697 | 0.263 | 1.259 | 531 | 0.199 | 0.969 | 490 | 0.237 | 0.813 |
| 2 | 465 | 0.145 | 0.794 | 701 | 0.206 | 1.211 | 526 | 0.183 | 0.912 | 405 | 0.171 | 0.621 |
| 3 | 504 | 0.210 | 0.882 | 643 | 0.199 | 1.169 | 514 | 0.199 | 0.900 | 395 | 0.162 | 0.623 |
| Pyrolysis Temperature (°C) | CC50 µg/mL (CI95%; R2) | ||
|---|---|---|---|
| HEC-1A | THP1 | MT-2 | |
| 500 | >1000 | >1000 | >1000 |
| 600 | >1000 | >1000 | >1000 |
| 700 | >1000 | >1000 | >1000 |
| 800 | ≈1000 | ≈1000 | 447.5 (269.0–765.0; 0.9012) |
| % APS | 500 | 600 | 700 | 800 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BET | Vmicro | Vmeso | BET | Vmicro | Vmeso | BET | Vmicro | Vmeso | BET | Vmicro | Vmeso | |
| (m2/g) | cm3/g | (m2/g) | cm3/g | (m2/g) | cm3/g | (m2/g) | cm3/g | |||||
| 0 | 534 | 0.119 | 1.456 | 679 | 0.158 | 1.348 | 562 | 0.270 | 1.133 | 427 | 0.139 | 0.600 |
| 0.3 | 554 | 0.003 | 1.131 | 687 | 0.235 | 1.402 | 551 | 0.129 | 1.126 | 316 | 0.131 | 0.607 |
| 0.5 | 594 | 0.028 | 1.233 | 669 | 0.173 | 1.407 | 459 | 0.082 | 0.985 | 281 | 0.109 | 0.615 |
| 1 | 546 | 0.056 | 1.147 | 665 | 0.226 | 1.376 | 398 | 0.071 | 0.844 | 243 | 0.094 | 0.614 |
| 2 | 517 | 0.255 | 1.059 | 653 | 0.189 | 1.354 | 325 | 0.047 | 0.707 | 230 | 0.083 | 0.521 |
| 3 | 537 | 0.186 | 1.100 | 626 | 0.178 | 1.365 | 323 | 0.082 | 0.751 | 202 | 0.015 | 0.499 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Illana, A.; Cazorla-Luna, R.; Notario-Pérez, F.; Ruiz-Caro, R.; Bedoya, L.M.; Veiga-Ochoa, M.D.; Rubio, J.; Tamayo, A. Amino Functionalized Micro-Mesoporous Hybrid Particles for the Sustained Release of the Antiretroviral Drug Tenofovir. Materials 2020, 13, 3494. https://doi.org/10.3390/ma13163494
Martin-Illana A, Cazorla-Luna R, Notario-Pérez F, Ruiz-Caro R, Bedoya LM, Veiga-Ochoa MD, Rubio J, Tamayo A. Amino Functionalized Micro-Mesoporous Hybrid Particles for the Sustained Release of the Antiretroviral Drug Tenofovir. Materials. 2020; 13(16):3494. https://doi.org/10.3390/ma13163494
Chicago/Turabian StyleMartin-Illana, Araceli, Raul Cazorla-Luna, Fernando Notario-Pérez, Roberto Ruiz-Caro, Luis Miguel Bedoya, Maria Dolores Veiga-Ochoa, Juan Rubio, and Aitana Tamayo. 2020. "Amino Functionalized Micro-Mesoporous Hybrid Particles for the Sustained Release of the Antiretroviral Drug Tenofovir" Materials 13, no. 16: 3494. https://doi.org/10.3390/ma13163494