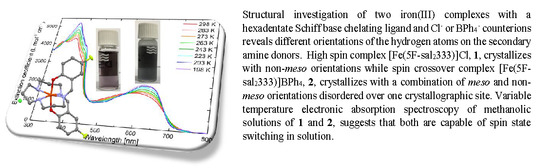
Anion Influence on Spin State in Two Novel Fe(III) Compounds: [Fe(5F-sal2333)]X
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis
2.3. Single-Crystal X-Ray Structure Determinations
2.4. Magnetic Measurements
2.5. UV-Vis and Electron Paramagnetic Resonance (EPR) Spectroscopy
3. Results
3.1. Synthetic Route
3.2. Structural Analysis
3.3. Magnetic Characterization
3.4. Solid State EPR Spectroscopy
3.5. UV-Vis Solution Studies
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Compound | [Fe(5F-sal2333)]Cl (1) | [Fe(5F-sal2333)]BPh4 (2) | [Fe(5F-sal2333)]BPh4 (2) |
|---|---|---|---|
| Empirical formula | C23H28N4O2F2ClFe | C47H48BN4O2F2Fe | C47H48BN4O2F2Fe |
| Formula weight | 521.79 | 805.55 | 805.55 |
| Crystal system | orthorhombic | triclinic | triclinic |
| Space group | Pccn | P−1 | P−1 |
| Crystal size (nm) | 0.195 × 0.114 × 0.033 | 0.3335 × 0.2632 × 0.2017 | 0.405 × 0.350 × 0.337 |
| a (Å) | 7.39810(6) | 10.7385(2) | 10.80774(8) |
| b (Å) | 16.3083(2) | 13.9687(3) | 14.0136(1) |
| c (Å) | 18.6359(2) | 14.7851(2) | 14.9232(2) |
| α (°) | 90 | 102.984(2) | 103.0383(7) |
| β (°) | 90 | 94.039(1) | 94.2739(6) |
| γ (°) | 90 | 109.415(2) | 107.6673(7) |
| V (Å3) | 2248.43(4) | 2012.64(6) | 2073.03(4) |
| Z | 4 | 2 | 2 |
| dcalc (g cm−3) | 1.541 | 1.329 | 1.291 |
| T (K) | 100(2) | 100(2) | 293(2) |
| µ (mm−1) | 6.871 | 0.429 | 0.416 |
| F(000) | 1084 | 846 | 846 |
| Limiting indices | h = ± 9, k = ± 20, l = ± 23 | h = ± 13, k = ± 17, l = ± 8 | h = ± 13, k = ± 17, l = ± 18 |
| Reflections collected/unique | 21831/2378 | 35866/8833 | 79115/7853 |
| R(int) | 0.0288 | 0.0315 | 0.0191 |
| Completeness to Θ (%) | 99.7 | 99.5 | 99.7 |
| Data/restraints/parameters | 2378/0/151 | 8833/0/578 | 7853/0/578 |
| GooF on F2 | 1.047 | 1.041 | 1.079 |
| Final R indices (I > 2σ (I)) | R1 = 0.0274, wR2 = 0.0760 | R1 = 0.0414, wR2 = 0.0928 | R1 = 0.0396, wR2 = 0.1068 |
| R indices (all data) | R1 = 0.0292, wR2 = 0.0777 | R1 = 0.0479, wR2 = 0.0963 | R1 = 0.0420, wR2 = 0.1085 |
| Largest diff. peak/hole (e−Å−3) | 0.255 and −0.457 | 0.332 and −0.723 | 0.292 and −0.584 |
| CCDC no. | 1884365 | 1884366 | 1884367 |
References
- Martinho, P.N.; Gildea, B.; Harris, M.M.; Lemma, T.; Naik, A.D.; Müller-Bunz, H.; Keyes, T.E.; Garcia, Y.; Morgan, G.G. Cooperative Spin Transition in a Mononuclear Manganese(III) Complex. Angew. Chem. Int. Ed. 2012, 51, 12597–12601. [Google Scholar] [CrossRef] [PubMed]
- Gildea, B.; Harris, M.M.; Gavin, L.C.; Murray, C.A.; Ortin, Y.; Müller-Bunz, H.; Harding, C.J.; Lan, Y.; Powell, A.K.; Morgan, G.G. Substituent Effects on Spin State in a Series of Mononuclear Manganese(III) Complexes with Hexadentate Schiff-Base Ligands. Inorg. Chem. 2014, 53, 6022–6033. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.G.; Murnaghan, K.D.; Müller-Bunz, H.; McKee, V.; Harding, C.J. A Manganese(III) Complex That Exhibits Spin Crossover Triggered by Geometric Tuning. Angew. Chem. Int. Ed. 2006, 45, 7192–7195. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Shakespeare, S.; Shepherd, H.J.; Harding, C.J.; Létard, J.F.; Desplanches, C.; Goeta, A.E.; Howard, J.A.K.; Powell, A.K.; Mereacre, V.; et al. A Symmetry-Breaking Spin-State Transition in Iron(III). Angew. Chem. Int. Ed. 2011, 50, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.; Gildea, B.; Müller-Bunz, H.; Harding, C.J.; Morgan, G.G. Co-Crystallisation of Competing Structural Modes in Geometrically Constrained Jahn–Teller Manganese(III) Complexes. Dalton Trans. 2012, 41, 14487–14489. [Google Scholar] [CrossRef] [PubMed]
- Martinho, P.N.; Kühne, I.A.; Gildea, B.; McKerr, G.; O’Hagan, B.; Keyes, T.E.; Lemma, T.; Gandolfi, C.; Albrecht, M.; Morgan, G.G. Self-Assembly Properties of Amphiphilic Iron(III) Spin Crossover Complexes in Water and at the Air–Water Interface. Magnetochemistry 2018, 4, 49. [Google Scholar] [CrossRef]
- Gandolfi, C.; Cotting, T.; Martinho, P.N.; Sereda, O.; Neels, A.; Morgan, G.G.; Albrecht, M. Synthesis and Self-Assembly of Spin-Labile and Redox-Active Manganese(III) Complexes. Dalton Trans. 2011, 40, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ferbinteanu, M.; Marinescu, C.; Dobrinescu, A.; Ling, Q.-D.; Huang, W. Case Study on a Rare Effect: The Experimental and Theoretical Analysis of a Manganese(III) Spin-Crossover System. Inorg. Chem. 2010, 49, 9839–9851. [Google Scholar] [CrossRef]
- Gildea, B.; Gavin, L.C.; Murray, C.A.; Müller-Bunz, H.; Harding, C.J.; Morgan, G.G. Supramolecular Modulation of Spin Crossover Profile in Manganese(III). Supramol. Chem. 2012, 24, 641–653. [Google Scholar] [CrossRef]
- Wang, S.; Xu, W.-T.; He, W.-R.; Takaishi, S.; Li, Y.-H.; Yamashita, M.; Huang, W. Structural Insights into the Counterion Effects on the Manganese(III) Spin Crossover System with Hexadentate Schiff-base Ligands. Dalton Trans. 2016, 45, 5676–5688. [Google Scholar] [CrossRef]
- Fitzpatrick, A.J.; Trzop, E.; Müller-Bunz, H.; Dîrtu, M.M.; Garcia, Y.; Collet, E.; Morgan, G.G. Electronic vs. Structural Ordering in a Manganese(III) Spin Crossover Complex. Chem. Commun. 2015, 51, 17540–17543. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.J.; Stepanovic, S.; Müller-Bunz, H.; Gruden-Pavlović, M.A.; García-Fernández, P.; Morgan, G.G. Challenges in Assignment of Orbital Populations in a High Spin Manganese(III) Complex. Dalton Trans. 2016, 45, 6702–6708. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, K.; Gildea, B.; Murray, C.; Harding, C.J.; Müller-Bunz, H.; Morgan, G.G. Lattice Effects on the Spin-Crossover Profile of a Mononuclear Manganese(III) Cation. Chem. Eur. J. 2012, 18, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, W.-R.; Ferbinteanu, M.; Li, Y.-H.; Huang, W. Tetragonally Compressed High-Spin Mn(III) Schiff Base Complex: Synthesis, Crystal Structure, Magnetic Properties and Theoretical Calculations. Polyhedron 2013, 52, 1199–1205. [Google Scholar] [CrossRef]
- Floquet, S.; Carmen Muñoz, M.; Rivière, E.; Clément, R.; Audière, J.-P.; Boillot, M.-L. Structural Effects on the Magnetic Properties of Ferric Complexes in Molecular Materials or a Lamellar CdPS3 Host Matrix. New J. Chem. 2004, 28, 535–541. [Google Scholar] [CrossRef]
- Pritchard, R.; Barrett, S.A.; Kilner, C.A.; Halcrow, M.A. The Influence of Ligand Conformation on the Thermal Spin Transitions in Iron(III) Saltrien Complexes. Dalton Trans. 2008, 3159–3168. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Kino, K.; Kida, S. X-Ray structural study of [Fe(saltrien)]X (X = Br·2H2O, BPh4 or PF6). Origin of Unusual Magnetic Behaviour of the Spin-Crossover Complex [Fe(saltrien)]PF6. J. Chem. Soc. Dalton Trans. 1987, 1957–1961. [Google Scholar] [CrossRef]
- Sinn, E.; Sim, G.; Dose, E.V.; Tweedle, M.F.; Wilson, L.J. Iron(III) Chelates with Hexadentate Ligands from Triethylenetetramine and b-diketones or Salicylaldehyde. Spin State Dependent Crystal and Molecular Structures of [Fe(acac)2trien]PF6 (S = 5/2), [Fe(acacCl)2trien]PF6 (S = 5/2), [Fe(sal)2trien]Cl·2H2O (S = 1/2). J. Am. Chem. Soc. 1978, 100, 3375–3390. [Google Scholar] [CrossRef]
- Martinho, P.N.; Harding, C.J.; Müller-Bunz, H.; Albrecht, M.; Morgan, G.G. Inducing Spin Crossover in Amphiphilic Iron(III) Complexes. Eur. J. Inorg. Chem. 2010, 2010, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, C.; Moitzi, C.; Schurtenberger, P.; Morgan, G.G.; Albrecht, M. Improved Cooperativity of Spin-Labile Iron(III) Centers by Self-Assembly in Solution. J. Am. Chem. Soc. 2008, 130, 14434–14435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinho, P.N.; Lemma, T.; Gildea, B.; Picardi, G.; Müller-Bunz, H.; Forster, R.J.; Keyes, T.E.; Redmond, G.; Morgan, G.G. Template Assembly of Spin Crossover One-Dimensional Nanowires. Angew. Chem. Int. Ed. 2012, 51, 11995–11999. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.J.C.; Coutinho, J.T.; Santos, I.C.; Pereira, L.C.J.; Waerenborgh, J.C.; da Gama, V. [Fe(nsal2trien)]SCN, a New Two-Step Iron(III) Spin Crossover Compound, with Symmetry Breaking Spin-State Transition and an Intermediate Ordered State. Inorg. Chem. 2013, 52, 3845–3850. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Šalitroš, I.; Trávníček, Z.; Moncoľ, J.; Fuess, H.; Ruben, M.; Linert, W. Anion Driven Modulation of Magnetic Intermolecular Interactions and Spin Crossover Properties in an Isomorphous Series of Mononuclear Iron(III) Complexes with a Hexadentate Schiff Base Ligand. CrystEngComm 2012, 14, 7015–7024. [Google Scholar] [CrossRef]
- Hayami, S.; Matoba, T.; Nomiyama, S.; Kojima, T.; Osaki, S.; Maeda, Y. Structures and Magnetic Properties of Some Fe(III) Complexes with Hexadentate Ligands: In Connection with Spin-Crossover Behavior. Bull. Chem. Soc. Jpn. 1997, 70, 3001–3009. [Google Scholar] [CrossRef]
- Ito, T.; Sugimoto, M.; Ito, H.; Toriumi, K.; Nakayama, H.; Mori, W.; Sekizaki, M. A Chelate Ring Size Effect on Spin States of Iron(III) Complexes with Hexadentate Ligands Derived from Salicylaldehyde and 4,8-Diazaundecane-1,11-diamine(3,3,3-tet) or 4,7-Diazadecane-1,10-diamine(3,2,3-tet), and their X-Ray Structures. Chem. Lett. 1983, 12, 121–124. [Google Scholar] [CrossRef]
- Kannappan, R.; Tanase, S.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. Low-Spin Iron(III) Schiff-Base Complexes with Symmetric Hexadentate Ligands: Synthesis, Crystal Structure, Spectroscopic and Magnetic Properties. Polyhedron 2006, 25, 1646–1654. [Google Scholar] [CrossRef]
- Fitzpatrick, A.J.; O’Connor, H.M.; Morgan, G.G. A Room Temperature Spin Crossover Ionic Liquid. Dalton Trans. 2015, 44, 20839–20842. [Google Scholar] [CrossRef]
- Seredyuk, M.; Gaspar, A.B.; Ksenofontov, V.; Galyametdinov, Y.; Verdaguer, M.; Villain, F.; Gütlich, P. Spin-Crossover and Liquid Crystal Properties in 2D cyanide-bridged FeII-MI/II Metalorganic Frameworks. Inorg. Chem. 2010, 49, 10022–10031. [Google Scholar] [CrossRef]
- Soyer, H.; Mingotaud, C.; Boillot, M.-L.; Delhaes, P. Spin Crossover of a Langmuir−Blodgett Film Based on an Amphiphilic Iron(II) Complex. Langmuir 1998, 14, 5890–5895. [Google Scholar] [CrossRef]
- Clark, R.C.; Reid, J.S. The Analytical Calculation of Absorption in Multifaceted Crystals. Acta Crystallogr. Sect. A 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]









| Bond Type | R-Sal2-222 | R-Sal2-232 | R-Sal2-323 | R-Sal2-333 |
|---|---|---|---|---|
| O | cis | cis | trans | trans |
| Namine | cis | cis | cis | cis |
| Nimine | trans | trans | cis | cis |
| Mn(III) | HS [3,7] | HS a | LS, HS, SCO [1,2,3,7,8,9,10,11,12,13] | HS [14] |
| Fe(III) | LS, HS, SCO [15,16,17,18,19,20,21,22,23,24] | LS, HS, SCO [4,24] | LS [24,25,26] | HS, SCO [24,25] |
| [Fe(5F-sal2333)]Cl (1) 100 K | Fe-Donor | [Fe(5F-sal2333)]BPh4 (2) 100 K | [Fe(5F-sal2333)]BPh4 (2) 293 K | |
|---|---|---|---|---|
| Fe-Ophenolate | 1.9426(9) | Fe–O(2) | 1.8521(14) | 1.8569(15) |
| Fe–O(1) | 1.8721(15) | 1.8801(16) | ||
| Fe-Nimine | 2.1398(11) | Fe–N(1) | 1.9480(15) | 1.9667(18) |
| Fe–N(4) | 1.9582(14) | 1.9778(16) | ||
| Fe-Namine | 2.1837(11) | Fe–N(3A) | 2.014(3) | 2.017(5) |
| Fe–N(2) | 2.0624(16) | 2.0734(19) | ||
| Fe–N(3B) | 2.134(4) | 2.162(7) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaresan, S.; Kühne, I.A.; Kelly, C.T.; Barker, A.; Salley, D.; Müller-Bunz, H.; Powell, A.K.; Morgan, G.G. Anion Influence on Spin State in Two Novel Fe(III) Compounds: [Fe(5F-sal2333)]X. Crystals 2019, 9, 19. https://doi.org/10.3390/cryst9010019
Sundaresan S, Kühne IA, Kelly CT, Barker A, Salley D, Müller-Bunz H, Powell AK, Morgan GG. Anion Influence on Spin State in Two Novel Fe(III) Compounds: [Fe(5F-sal2333)]X. Crystals. 2019; 9(1):19. https://doi.org/10.3390/cryst9010019
Chicago/Turabian StyleSundaresan, Sriram, Irina A. Kühne, Conor T. Kelly, Andrew Barker, Daniel Salley, Helge Müller-Bunz, Annie K. Powell, and Grace G. Morgan. 2019. "Anion Influence on Spin State in Two Novel Fe(III) Compounds: [Fe(5F-sal2333)]X" Crystals 9, no. 1: 19. https://doi.org/10.3390/cryst9010019