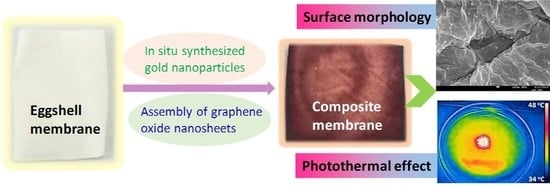
Sunlight-Driven Photothermal Effect of Composite Eggshell Membrane Coated with Graphene Oxide and Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. In-Situ Synthesis of AuNPs on ESM
2.4. Assembly of GO on ESM
2.5. Photothermal Measurement of ESM
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, L.; Tan, Y.L.; Wang, J.Y.; Xu, W.C.; Yuan, Y.; Cai, W.S.; Zhu, S.N.; Zhu, J. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, M.A. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Shen, D.; Zhou, L.; Li, W.; Li, X.; Yao, C.; Wang, R.; El-Toni, A.M.; Zhang, F.; Zhao, D. Spatially confined fabrication of core-shell gold nanocages@mesoporous silica for near-infrared controlled photothermal drug release. Chem. Mater. 2013, 25, 3030–3037. [Google Scholar] [CrossRef]
- Ricciardi, L.; Sancey, L.; Palermo, G.; Termine, R.; De Luca, A.; Szerb, E.I.; Aiello, I.; Ghedini, M.; Strangi, G.; La Deda, M. Plasmon-mediated cancer phototherapy: The combined effect of thermal and photodynamic processes. Nanoscale 2017, 9, 19279–19289. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Chang, C.; Yang, C.; Liu, Y.M.; Tao, P.; Song, C.Y.; Shang, W.; Wu, J.B.; Deng, T. Efficient solar-thermal energy harvest driven by interfacial plasmonic heating-assisted evaporation. ACS Appl. Mater. Interfaces 2016, 8, 23412–23418. [Google Scholar] [CrossRef]
- Smith, J.G.; Faucheaux, J.A.; Jain, P.K. Plasmon resonances for solar energy harvesting: A mechanistic outlook. Nano Today 2015, 10, 67–80. [Google Scholar] [CrossRef]
- Metwally, K.; Mensah, S.; Baffou, G. Fluence threshold for photothermal bubble generation using plasmonic nanoparticles. J. Phys. Chem. C 2015, 119, 28586–28596. [Google Scholar] [CrossRef]
- Palermo, G.; Ritacco, T.; Aceti, D.M.; Pezzi, L.; Giocondo, M.; De Luca, A. Photo-thermal effects in 1D gratings of gold nanoparticles. Crystals 2017, 7, 14. [Google Scholar] [CrossRef]
- Pezzi, L.; Palermo, G.; Veltri, A.; Cataldi, U.; Bürgi, T.; Ritacco, T.; Giocondo, M.; Umeton, C.; De Luca, A. Photo-thermal study of a layer of randomly distributed gold nanoparticles: From nano-localization to macro-scale effects. J. Phys. D Appl. Phys. 2017, 50, 435302. [Google Scholar] [CrossRef]
- Palermo, G.; Pagnotto, D.; Ricciardi, L.; Pezzi, L.; La Deda, M.; De Luca, A. Thermoplasmonic effects in gain-assisted nanoparticle solutions. J. Phys. Chem. C 2017, 121, 24185–24191. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.Z.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, X.J.; Ren, J.S.; Qu, K.G.; Qu, X.G. Using graphene oxide high near-infrared absorbance for photothermal treatment of alzheimer’s disease. Adv. Mater. 2012, 24, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhao, L.; Chen, H.; He, X.; Li, W.X.; Du, X.; Sun, Z.M.; Zhang, T.; Shen, Y. Graphene oxide foam fabricated with surfactant foaming method for efficient solar vapor generation. J. Mater. Sci. 2019, 54, 12782–12793. [Google Scholar] [CrossRef]
- Xu, Y.R.; Hu, X.L.; Guan, P.; Du, C.B.; Tian, Y.; Ding, S.C.; Li, Z.L.; Yan, C.R. A novel controllable molecularly imprinted drug delivery system based on the photothermal effect of graphene oxide quantum dots. J. Mater. Sci. 2019, 54, 9124–9139. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Tang, M.; Zhou, L.; Zhu, B.; Zhu, S.; Zhu, J. Graphene oxide-based efficient and scalable solar desalination under one sun with a confined 2D water path. Proc. Natl. Acad. Sci. USA 2016, 113, 13953–13958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.W.; Deng, W.J.; Yang, F.; Wu, Z.Q.; Huang, M.T.; Gu, M.Y. Gold nanoparticles decorated graphene oxide/nanocellulose paper for NIR laser-induced photothermal ablation of pathogenic bacteria. Carbohydr. Polym. 2018, 198, 206–214. [Google Scholar] [CrossRef]
- Xu, C.; Ma, B.; Peng, J.L.; Gao, L.; Xu, Y.H.; Huan, Z.G.; Chang, J. Tricalcium silicate/graphene oxide bone cement with photothermal properties for tumor ablation. J. Mat. Chem. B 2019, 7, 2808–2818. [Google Scholar] [CrossRef]
- Cao, Y.; Hassan, M.; Cheng, Y.; Chen, Z.R.; Wang, M.; Zhang, X.Z.; Haider, Z.S.; Zhao, G. Multifunctional Photo-and Magnetoresponsive Graphene Oxide-Fe3O4 Nanocomposite-alginate hydrogel platform for ice recrystallization inhibition. ACS Appl. Mater. Interfaces 2019, 11, 12379–12388. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Johnson, O.; Huang, J.; Feng, T.; Yang, C.Q.; Liu, Z.X.; Chen, W. Enhancing the photothermal conversion efficiency of graphene oxide by doping with NaYF4: Yb, Er upconverting luminescent nanocomposites. Mater. Res. Bull. 2018, 106, 365–370. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhang, J.; Wang, P.; Li, C.P.; Xu, X.L.; Jin, Y.D. Bifunctional plasmonic colloidosome/graphene oxide-based floating membranes for recyclable high-efficiency solar-driven clean water generation. Nano Res. 2018, 11, 3854–3863. [Google Scholar] [CrossRef]
- Mittal, A.; Teotia, M.; Soni, R.K.; Mittal, J. Applications of egg shell and egg shell membrane as adsorbents: A review. J. Mol. Liq. 2016, 223, 376–387. [Google Scholar] [CrossRef]
- Daraei, H.; Mittal, A.; Mittal, J.; Kamali, H. Optimization of Cr (VI) removal onto biosorbent eggshell membrane: Experimental & theoretical approaches. Desalin. Water Treat. 2014, 52, 1307–1315. [Google Scholar]
- Chen, W.; Li, B.; Xu, C.; Wang, L. Chemiluminescence flow biosensor for hydrogen peroxide using DNAzyme immobilized on eggshell membrane as a thermally stable biocatalyst. Biosens. Bioelectron. 2009, 24, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Messens, W.; Grijspeerdt, K.; Herman, L. Eggshell characteristics and penetration by Salmonella enterica serovar Enteritidis through the production period of a layer flock. Br. Poult. Sci. 2005, 46, 694–700. [Google Scholar] [CrossRef]
- Kaweewong, K.; Garnjanagoonchorn, W.; Jirapakkul, W.; Roytrakul, S. Solubilization and identification of hen eggshell membrane proteins during different times of chicken embryo development using the proteomic approach. Protein J. 2013, 32, 297–308. [Google Scholar] [CrossRef]
- Jun, H.J.; Oh, K.-H.; Yoo, J.; Han, W.-G.; Chang, J.; Jung, H.H.; Choi, J. A new patch material for tympanic membrane perforation by trauma: The membrane of a hen egg shell. Acta Otolaryngol. (Stockh.) 2014, 134, 250–254. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Suso, H.-P.; Maqbool, A.; Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 95, 192–203. [Google Scholar] [CrossRef]
- Ruff, K.J.; Winkler, A.; Jackson, R.W.; DeVore, D.P.; Ritz, B.W. Eggshell membrane in the treatment of pain and stiffness from osteoarthritis of the knee: A randomized, multicenter, double-blind, placebo-controlled clinical study. Clin. Rheumatol. 2009, 28, 907–914. [Google Scholar] [CrossRef]
- Ruff, K.J.; DeVore, D.P.; Leu, M.D.; Robinson, M.A. Eggshell membrane: A possible new natural therapeutic for joint and connective tissue disorders. Results from two open-label human clinical studies. Clin. Interv. Aging 2009, 4, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.M.; Xu, Z.L.; Yang, M.Y.; Tang, B.; Wang, X.G. Functionalization of cotton fabrics through thermal reduction of graphene oxide. Appl. Surf. Sci. 2017, 393, 441–448. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ikawa, N.; Ozimek, L. Chemical composition of chicken eggshell and shell membranes. Poult. Sci. 2003, 82, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Guo, Z.-X.; Zhang, L.-X.; Yu, J.; Li, Q. Soluble eggshell membrane protein: Preparation, characterization and biocompatibility. Biomaterials 2004, 25, 4591–4599. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Sun, L.; Kaur, J.; Yu, Y.; Wang, X.G. In-situ synthesis of gold nanoparticles for multifunctionalization of silk fabrics. Dyes Pigm. 2014, 103, 183–190. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, Z.; Wang, Y.; Chen, D.; Yang, D. Photocatalytic properties of porous C-doped TiO2 and Ag/C-doped TiO2 nanomaterials by eggshell membrane templating. J. Nanopart. Res. 2009, 11, 375–384. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Zhang, L.; Lv, Y. Microwave-assisted synthesis of carbon nanodots through an eggshell membrane and their fluorescent application. Analyst 2012, 137, 5392–5397. [Google Scholar] [CrossRef]
- Zheng, B.; Qian, L.; Yuan, H.; Xiao, D.; Yang, X.; Paau, M.C.; Choi, M.M.F. Preparation of gold nanoparticles on eggshell membrane and their biosensing application. Talanta 2010, 82, 177–183. [Google Scholar] [CrossRef]
- Balakumar, V.; Prakash, P. A facile in situ synthesis of highly active and reusable ternary Ag-PPy-GO nanocomposite for catalytic oxidation of hydroquinone in aqueous solution. J. Catal. 2016, 344, 795–805. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Tang, B.; Zhou, J.; Zhao, H.; Chen, W.; Wang, J. Sunlight-Driven Photothermal Effect of Composite Eggshell Membrane Coated with Graphene Oxide and Gold Nanoparticles. Appl. Sci. 2019, 9, 4384. https://doi.org/10.3390/app9204384
Wang L, Tang B, Zhou J, Zhao H, Chen W, Wang J. Sunlight-Driven Photothermal Effect of Composite Eggshell Membrane Coated with Graphene Oxide and Gold Nanoparticles. Applied Sciences. 2019; 9(20):4384. https://doi.org/10.3390/app9204384
Chicago/Turabian StyleWang, Ling, Bin Tang, Ji Zhou, Hai Zhao, Wu Chen, and Jinfeng Wang. 2019. "Sunlight-Driven Photothermal Effect of Composite Eggshell Membrane Coated with Graphene Oxide and Gold Nanoparticles" Applied Sciences 9, no. 20: 4384. https://doi.org/10.3390/app9204384