Taiwo Ayodele Aderinola
Phone: +2347036569677
E-Mail: taaderinola@futa.edu.ng
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 4
Page No: 720-728
Taiwo Ayodele Aderinola
Phone: +2347036569677
E-Mail: taaderinola@futa.edu.ng
Taiwo Ayodele Aderinola1,2*, Adeola Monisola Alashi2, Tayo Nathaniel Fagbemi1, Victor Ndigwe Enujiugha1, Rotimi Emmanuel Aluko2
1Department of Food Science and Technology, The Federal University of Technology, Akure, P.M.B. 704, Akure, Nigeria
2 Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, MB R3T 2N2, Canada
Hakuto Kageyama(kageyama@meijo-u.ac.jp)
Xuan-Ming Zhang(lenghanxing_0@163.com)
Azzurra Stefanucci(a.stefanucci@unich.it)
Paulo Vale(pvale@ipma.pt)
Taiwo Ayodele Aderinola, MORINGA OLEIFERA FLOUR PROTEIN FRACTIONS AS FOOD INGREDIENTS WITH ANTIOXIDANT PROPERTIES(2019)SDRP Journal of Food Science & Technology 4(4)
Moringa oleifera meal proteins were extracted by using salt, water and alkaline precipitation to obtain globulins (GLO), albumins (ALB), and iso-electric precipitated (ISO) isolates. All the samples were tested for antioxidant properties using 2, 2diphenyl-1-picrylhydrazyl (DPPH), hydroxyl radical scavenging activity (HRSA), ferric reducing antioxidant power (FRAP) and inhibition of metal ion chelation assays. The amino acid compositions of the samples, though fairly comparable, the albumin fraction in particular showed considerable inhibitory potentials against the DPPH (53.02%) and hydroxyl radical (44.21%). None of the samples however had any significant metal chelating ability since the only sample (globulin) with activity had less than 5% metal chelation activity. With the results obtained in the study, it was concluded that the albumin and the flour possess some level of inhibitory potentials against free radicals which could present them as useful antioxidant agents.
Key words: Moringa oleifera seed; protein isolates; antioxidant activity; amino acids
Recently, plant foods, including legume or pulse seeds are gaining increasing attention due to the bioactivities of their proteins and peptides which have been shown to possess from moderate to high antioxidant potentials against the attacks of free radicals (Aderinola et al. 2018a; b; Mahomoodally et al. 2019; Udenigwe and Aluko 2012). Moringa oleifera (MF) is a unique and a very special tree having almost every part of its tree useful for food or other beneficiary properties. It has been described with the taste of its roots as horse-radish tree or with the shape of its pods as drumstick tree (Lalas and Tsaknis 2002). The different parts such as the leaves, fruits, flower and the immature pods have been utilized in various forms including teas, soups or parts of other meals. Specifically, the seeds are eaten as peas, roasted and consumed like nuts (Stevens et al. 2013). Moringa oleifera seed has protein content with essential amino acid compositions, and also good fatty acid compositions (Ogunsina et al. 2011). The new approach to finding protective molecules that provide maximum protection to body organs with easy availability and minimal side effects is going on throughout the world. Many researchers have reported that proteins isolated from plant sources show antioxidant activities (Huang et al. 2010; Li et al. 2008; Tang et al. 2009). Different bioactivities and physiological properties have been exhibited by different food proteins some of which include antioxidative, antimicrobial, opoid, immunomodulatory mineral binding and antihypertensive (Korhonen and Pihlanto 2003; Luisi et al. 2018). While numerous research have been carried out on Moringa oleifera roots, leaves, seeds and oil (Anhwange et al. 2004; Atawodi et al. 2010; Mbah et al. 2012), there is little information on the antioxidant properties of Moringa oleifera seed protein fractions including isolates, albumins and globulins. The objective of this study therefore was to evaluate the antioxidant properties of Moringa oleifera seed proteins that can be used as ingredients in the functional food industry
Materials
Moringa oleifera seeds were purchased from Oba market, Akure, Ondo State, Nigeria. 2,2-diphenyl-1-picrylhydrazyl (DPPH), glutathione reduced (GSH), were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other analytical grade reagents were purchased from Fisher Scientific (Oakville, ON, Canada).
Sample preparation
Moringa seed flour (MF) was obtained from the seed by grinding the seed with coffee grinder (Cuisinant, DCG 12BCC, China) and defatted with acetone (1:10 w/v) under continuous stirring for 1 h allowed to sediment, filtered, re-dispersed in the same volume of acetone and the process repeated for another 1 h. After decanting the excess acetone, the defatted MF was air dried under a fume cupboard and ground to obtain the Moringa oleifera seed meal (MSM).
Isolation of protein fractions: iso-electric precipitated, albumin and globulin
Moringa oleifera seed iso-electric precipitated protein (ISO) fractions was obtained through a previously described method (Aluko 2004) with some modifications. In brief, was dispersed in 0.1 M NaOH (1:20, w/v), mixed for 1 h and centrifuged at room temperature (8000 g for 1 h). The supernatant was adjusted to pH 5 and kept in the refrigerator (8-10°C) for 8 – 12 h to precipitate. Later, centrifuged at 4°C and the precipitate was freeze dried. Moringa seed protein globulin and albumin fractions were obtained through the process of dialysis. Briefly, MSM was dispersed in 0.5M NaCl for 1 h with continuous stirring followed by centrifugation (8000 g, 60 min at 4°C). The supernatant was clarified with Whatman No 1 filter paper and the residue was discarded. The filtrate was dialyzed against water for 5 days at 4 °C using the 6-8 kDa MWCO dialysis tubing and the dialysis water was changed at least 3 times daily. Thereafter, the content of the dialysis tube was centrifuged (8000 g, 60 min at 4 °C) and the supernatant was collected as the albumin fraction. The precipitate was washed with distilled water and centrifuged under similar conditions. The precipitate was collected as the globulin protein fraction. Both fractions were freeze dried and stored at -20 °C until needed.
Determination of amino acid composition
The amino acid profiles of the samples were determined using an HPLC system, after samples were hydrolyzed with 6 M HCl (Bidlingmeyer et al. 1984). The cysteine and methionine contents were determined after performing acid oxidation (Gehrke et al. 1985) while the tryptophan content was determined after alkaline hydrolysis (Landry and Delhaye 1992).
Determination of antioxidant properties:
DPPH radical-scavenging activity
The scavenging activity of samples against the DPPH radical was determined using a previously described method (Aluko and Monu 2003) with slight modifications for a 96-well clear flat-bottom plate. Samples were dissolved in 0.1 M sodium phosphate buffer, pH 7.0 containing 1% (w/v) Triton X-100. DPPH was dissolved in methanol to a final concentration of 100 µM. Peptide samples (100 µL) were mixed with 100 µL of the DPPH●+ solution in the 96-well plate to a final assay concentration of 1.0 mg/ml and incubated at room temperature in the dark for 30 min. The absorbance values of the control (Ac) and samples (As) were measured at 517 nm. The control consisted of buffer in place of the peptide sample while GSH was used as the positive control. The percent DPPH radical scavenging activity of the samples was determined using the following equation:
DPPH Radical Scavenging Activity (%)= (Ac-As)/Ac
Where Ac and As are the absorbance of control and sample, respectively.
Ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power of samples was measured according to a previously described method (Benzie and Strain 1996) with some modifications for a microplate reader. Briefly, the FRAP reagent was freshly prepared by mixing 300 mM acetate buffer (sodium acetate buffer, pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl and 20 mM ferric chloride in a ratio 5:1:1 (v/v) before evaluation. Two hundred microlitres (200 µL) of FRAP reagent (preheated to 37°C) was added to 40 µL of sample or GSH in a 96 well microplate. Absorbance at 593 nm was measured relative to a reagent blank. Ferrous sulphate (conc: 0.0625-1 mM) was used to prepare a standard curve and the results of the samples were expressed as mmol Fe2+ reduced.
Chelation of metal ions
The metal chelating activity was measured using a modification of a previously described method (Xie et al. 2008). Five hundred microlitres (500 µL) of peptide sample solution or GSH (final assay concentration of 1 mg/ml) was combined with 25 µL of 2 mM FeCl2 and 925 µL double distilled water in a reaction tube. Fifty microlitre (50 µL) of ferrozine solution (5 mM) was added and mixed thoroughly. The mixture was then allowed to stand at room temperature for 10 min and an aliquot of 200 µL was pipetted into a clear bottom 96-well plate. A control was also conducted by replacing the sample with 500 µL of double distilled water. The absorbance values of control (Ac) and sample (As) at 562 nm were measured using a spectrophotometer. Percentage chelating effect (%) was calculated using the following equation:
Metal ion chelating activity (%)= (Ac-As)/Ac ×100
Hydroxyl radical scavenging assay
The hydroxyl radical scavenging assay was modified based on a previously described method (Girgih et al. 2011). Sample or GSH and 1, 10-phenanthroline (3 mM) were each separately dissolved in 0.1 M phosphate buffer (pH 7.4) while FeSO4 (3 mM) and 0.01% hydrogen peroxide were each separately dissolved in distilled water. An aliquot (50 µL) of sample or GSH (equivalent to a final assay concentration of 1 mg/ml) or buffer (control) was first added to a clear, flat bottom 96-well plate followed by additions of 50 µL of 1, 10-phenanthroline and 50 µL of FeSO4. To initiate the Fenton reaction in the wells, 50 µL of hydrogen peroxide (H2O2) solution was added to the mixture. The absorbance of the mixtures was measured at 536 nm every 10 min for a period of 1 h at 37 °C with continuous shaking. The hydroxyl radical scavenging activity was calculated as follows based on the changes in absorbance (ΔA):
Hydroxyl radical scavenging activity (%)= (∆A/min[control-∆A/minsample ])/(∆A/min [control ] )×100
Statistical analyses
Means of triplicate results ± standard deviation (except the amino acid composition) were analyzed using the one way analysis of variance with SPSS version 22.0 and the means were separated with Duncan’s Multiple Range (DMR) test at p<0.05.
Amino acid composition
One of the factors reported to be responsible for the antioxidant properties of peptides is their amino acid compositions (Malomo et al. 2014). The amino acid compositions of the Moringa oleifera seed flour and isolated proteins- albumin, globulin, and ISO isolate are shown in Table 1. The protein isolation process did not significantly alter the constituent amino acid since no major changes were observed in the amino acid composition of the Moringa oleifera seed protein fractions compared to the precursor flour (Moringa flour, MF) except for the albumin fraction (Gly, Lys and Arg). This trend was also observed between hemp seed protein isolate and its protein meal (Malomo et al. 2014). Generally, the seed flour and the proteins showed high contents of glutamic acid, glutamine (GLX), glycine and arginine. The combined summary of the compositions reveals the presence of more hydrophobic amino acids (HAA) and the negatively charged amino acids (NCAA) in the samples. The peculiar functions of some of these amino acids, especially, Tyr, Met, His and Lys in improving antioxidant properties of peptides have been reported (Samaranayaka and Li-chan 2011; Udenigwe and Aluko 2012). Also, some aromatic amino acid with large side group such as His (imidazole group) and Trp (indolic group) contribute to the antiradical potency of peptides as they act as hydrogen donators due to the special group they possess in their side chain (Nam et al. 2008). The interaction of the peptides with lipids or entry into target organs can be enhanced by their hydrophobic properties which help in promoting the antioxidant effects of the peptides (Sarmadi and Ismail 2010). Furthermore, hydrophobicity of protein, which helps to improve their solubility in lipid medium, has been reported to also improve their antioxidant potentials (Rajapakse et al. 2005). The Moringa seed flour and the protein isolates also show high content of the essential amino acids (EAA) which is an indication that they possess good nutritional values.
Table 1: Amino acid composition of Moringa oleifera seed flour and protein fractions
|
Amino acids |
Moringa flour |
Albumin |
Globulin |
ISO |
|
ASX |
4.96 |
6.72 |
3.53 |
6.27 |
|
THR |
2.88 |
4.18 |
2.29 |
3.35 |
|
SER |
3.71 |
5.38 |
3.28 |
3.19 |
|
GLX |
22.93 |
18.64 |
26.52 |
25.70 |
|
PRO |
6.69 |
5.28 |
6.57 |
5.90 |
|
GLY |
5.73 |
15.06 |
4.02 |
5.98 |
|
ALA |
4.52 |
3.85 |
4.10 |
4.41 |
|
CYS |
5.02 |
4.22 |
5.67 |
3.42 |
|
VAL |
3.99 |
3.45 |
3.74 |
4.46 |
|
MET |
2.10 |
1.39 |
2.53 |
1.93 |
|
ILE |
3.30 |
2.60 |
3.19 |
3.00 |
|
LEU |
6.13 |
4.59 |
5.94 |
6.33 |
|
TYR |
1.97 |
3.36 |
1.71 |
2.27 |
|
PHE |
4.50 |
3.45 |
4.10 |
5.35 |
|
HIS |
2.70 |
2.39 |
2.50 |
2.95 |
|
LYS |
1.83 |
5.23 |
0.45 |
1.29 |
|
ARG |
16.05 |
9.48 |
19.33 |
13.19 |
|
TRP |
0.97 |
0.75 |
0.53 |
0.99 |
|
AAA |
7.45 |
7.56 |
6.33 |
8.61 |
|
HAA |
39.20 |
32.93 |
38.08 |
38.07 |
|
PCAA |
20.58 |
17.09 |
22.28 |
17.43 |
|
NCAA |
27.89 |
25.36 |
30.05 |
31.97 |
|
SCAA |
7.12 |
5.61 |
8.20 |
5.35 |
|
EAA |
44.45 |
37.49 |
44.60 |
42.85 |
HAA, hydrophobic amino acids- alanine, valine, isoleucine, leucine, tryrosine, phenylalanine, tyrptophan, proline, methionine and cysteine
PCAA- positively charged amino acids- arginine, histidine and lysine
NCAA- negatively charged amino acids- ASX (aspargine +aspartic acid) and GLX (glutamine+glutamic acid)
AAA- aromatic amino acids- phenylalanine, tryptophan and tyrosine
SCAA- sulphur containing amino acids- cysteine and methionine
EAA – essential amino acids – threonine, valine, methionine, leucine, isoleucine, phenylalanine, histidine, lysine, arginine and tryptophan
Antioxidant properties of Moringa protein fractions
One of the most important means by which oxidative stress is prevented is through neutralization of free radicals. Scavenging of free radicals by antioxidants has been identified as a mode of operation of antioxidants against the attacks of free radicals. Since there are different types of free radicals produced from different sources and with different level of activities, different methods of determination are usually employed to measure the effectiveness of an antioxidant against a particular free radical. The different antioxidant tests carried out in this study include: DPPH, FRAP, MC and OH scavenging assays.
DPPH radical scavenging assay
DPPH radical scavenging assay is a commonly used antioxidant assay to measure the radical scavenging ability of a sample. It’s a stable free radical with maximum absorbance at 517 nm in methanol/ethanol. It has a proton-accepting tendency; so when there is a compound that has a free proton to donate, such as antioxidants, the DPPH free radical is scavenged leading to reduction in the measured absorbance (Girgih et al. 2011). The antioxidant property of such a compound is thus expressed as its ability to scavenge DPPH radicals. Figure 1 shows the radical scavenging ability of the isolated proteins (albumin, ALB; globulin, GLO and iso-electric precipitated, ISO) and MF. While there were significant differences (p<0.05) among the samples, the inhibitory potential of the ISO was significantly lower at 2.45% (p<0.05) than the other fractions. Although all the sample had high and similar hydrophobic amino acid contents, the alkaline soluble fractions of Moringa proteins were not able to scavenge the DPPH●+. The observed difference in the antioxidant potentials of the other protein ISO and GLO fractions may therefore be due to the specific location of these hydrophobic amino acids in protein sequence and the folding of the amino acid chains (Li et al. 2008; Pownall et al. 2010). The results show that the albumin and the MF possess more electron donating capability that can react with the DPPH●+, terminating the chain of radical reaction by converting them into a more stable product.
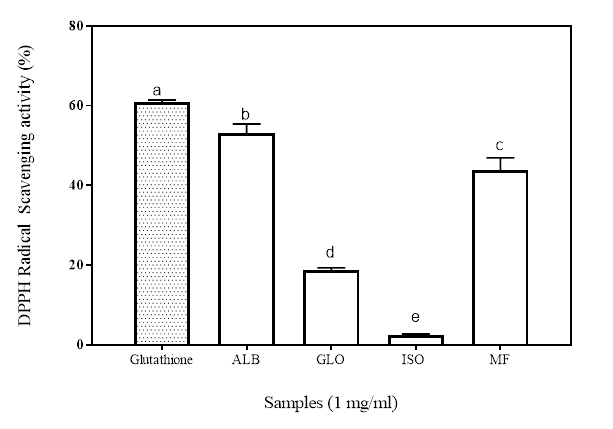
Figure 1: Percentage of DPPH radical inhibition of Moringa seed flour (MF) and its albumin (ALB), globulin (GLO) and isoelectric precipitated protein (ISO) fractions. Bars with different letters have mean values (n=3) that are significantly different (p<0.05)
Ferric reducing antioxidant power (FRAP)
The result of the ferric reducing antioxidant power (FRAP) of the protein isolates and the flour is shown in Figure 2. The FRAP is one of the means of evaluating the electron donating potential of an antioxidant compound whereby the Fe3+/ferric cyanide complex is reduced to the ferrous (Fe2+/) form (Yıldırım et al. 2001). There’s high correlation of the reducing ability of a compound to its antioxidant potentials (Gülçin et al. 2012). Some studies have also confirmed the direct correlation between antioxidant properties and the reducing properties of bioactive compounds (Wang et al. 2007). During the assay for reducing power, an antioxidant probe accepts electron from the antioxidant analyte (e.g. peptides) and becomes converted into the reduced probe which is colored (Berker et al. 2007). The protein fractions showed varied level of reducing potentials with the albumin having significantly higher (p<0.05) FRAP activity (0.20 mM Fe2+/mg sample) than GLO, ISO and MF at (0.04, 0.02, and 0.06 mM Fe2+/mg sample, respectively). The positive control, GSH (0.11, Fe2+/mg was analyzed at 0.125 mg/ml final assay concentration). The GSH value obtained here is significantly higher than all the protein fractions except ALB because it was analyzed at a lower concentration to the protein fractions. Some authors have also reported the ferric reducing abilities of some protein samples (Girgih et al. 2011; Li et al. 2008). These values are however comparable to some of these reported values. While Mundi and Aluko (2014) have attributed the presence of such amino acids as Leu, Lys, Met, Tyr, Ile, His and Trp with high reducing power, You et al (2009) have suggested that the concentration of Tyr, Met, His, Lys and Trp might be responsible for observed high reducing power. Therefore, Moringa seed flour and its protein fractions may provide a good ferric reducing capacity due to its high hydrophobic and positively branched chain amino acid content.
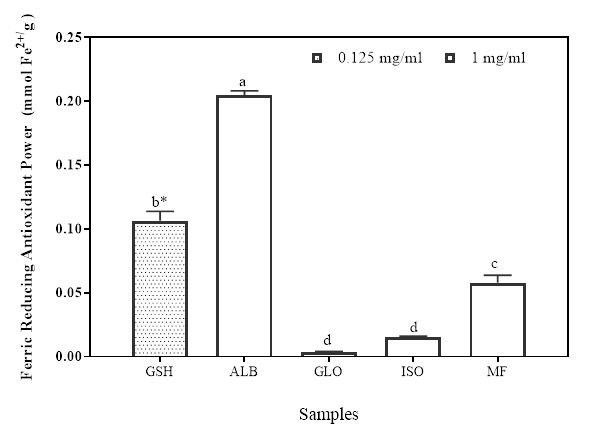
Figure 2: Ferric reducing antioxidant power of Moringa seed flour (MF) and its albumin (ALB), globulin (GLO) and isoelectric precipitated protein (ISO) fractions. Bars with different letters or star have mean values (n=3) that are significantly different (p<0.05) for both FRAP activity and assay concentration, respectively.
Hydroxyl radical scavenging ability
The hydroxyl radical scavenging ability of the isolated proteins is shown in Figure 3. According to Saiga et al (2003), protracted and severe oxidative stress in human can lead to the initiation or promotion of large number of chronic disease conditions. The hydroxyl radical (●OH) is generated from the conversion of superoxide and hydrogen peroxide as well as the metal catalyzed process. Indeed, that can lead to the oxidation of virtually all organic cell constituents including the DNA, lipids and proteins (Du and Gebicki 2004). A chain of reactions causing several damages is initiated when ●OH reacts with target macromolecules that are essential for normal and proper functioning of the cell components (Mundi and Aluko 2014). The Moringa seed globulin fractions was not active against the hydroxyl radicals. The albumin and the iso-electric precipitated, which were not significantly different (p<0.05) on the other hand had about 44.21% and 42.98% hydroxyl radical scavenging inhibition, respectively, while GSH was 88.47%. The hydroxyl radical scavenging ability of a sample is as a result of the combined effect of the reducing power, donation of hydrogen atoms and the scavenging of active oxygen (Yen and Hsieh 1995). This results suggest that Moringa oleifera seed albumin and iso-electric precipitated fractions may provide hydroxyl radical scavenging ability in food systems, thereby helping to offer a defensive shield against the hydroxyl radical which has been implicated as one of the major causative agents in aging and in many other chronic diseases within the body (Saiga et al. 2003). The negative value obtained for the GLO suggests it potentials as prooxidative agent. While generation of prooxidants could lead oxidative stress, its beneficial effects, especially as anticancer had also been reported (Chedea et al. 2014, Tsukada et al. 2016).
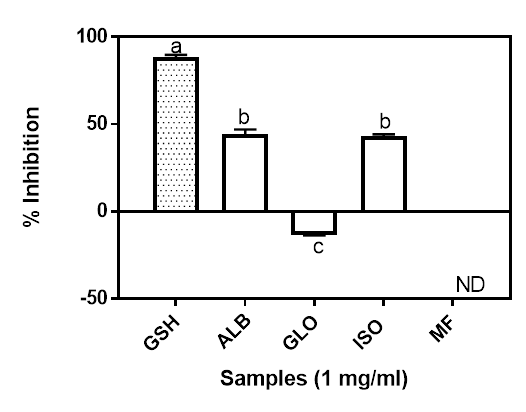
Figure 3: Hydroxyl radical scavenging ability of Moringa seed flour (MF) and its albumin (ALB), globulin (GLO) and isoelectric precipitated protein (ISO) fractions. Bars with different letters have mean values (n=3) that are significantly different (p<0.05). ND: Not Determined
Metal chelating activity
Iron has been reported to be involved in many pathways in a number of degenerative diseases (Mundi and Aluko 2014). The highly reactive hydroxyl radical can be generated from the redox-active Fe (II) when reacted with H2O2 through the Fenton reaction. Production of high level ●OH may lead to the onset of various oxidant-induced metabolic disorders. This may explain the critical link between the level of Fe(II) and oxidative stress in human body (Mundi and Aluko 2014). The metal chelating activity of the isolated proteins and the Moringa flour is shown in Figure 4. Only the globulin showed positive metal chelating activity at 4.12% which was not significantly different from the control sample GSH (3.81%) at 1 mg/ml final assay concentration. All the other fractions had negative values, suggesting that they exhibited prooxidative metal chelating activities. The presence of some side group such as amino and carboxyl groups of the acidic (Gly and Asx) and basic (Lys, His and Arg) amino acids have been reported to be involved in chelating metal ions (Saiga et al. 2003).
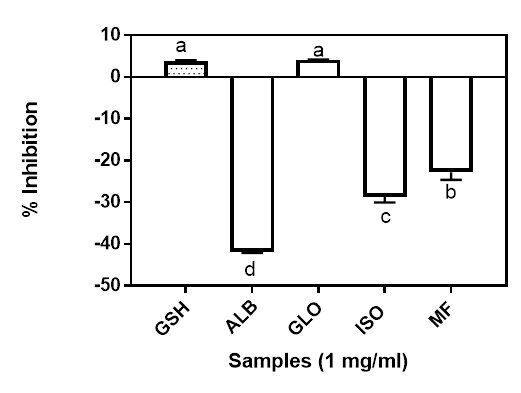
Figure 4: Metal chelating ability of Moringa seed flour (MF) and its albumin (ALB), globulin (GLO) and isoelectric precipitated protein (ISO) fractions. Bars with different letters have mean values (n=3) that are significantly different (p<0.05).
Moringa oleifera seed flour and protein isolates (albumin, globulin and isolate) were tested for their in-vitro antioxidant properties in this study. The results showed that Moringa oleifera seed flour and protein fractions, especially the albumin fraction could serve as a potential ingredient in the functional food industry as it possessed considerably higher free radical scavenging activities for all the assays tested but showed a prooxidative activity for metal ion activity as it was unable to chelate metal ion as were all other fractions.
Taiwo A. Aderinola is a beneficiary of funding for this study, provided by the Nigerian Tertiary Education Trust Fund (TETFund). Dr. R. E. Aluko’s research is funded by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Aderinola, T.A., Fagbemi, T.N., Enujiugha, V.N., Alashi, A.M. and Aluko, R.E. 2018. Amino acid composition and antioxidant properties of Moringa oleifera seed protein isolate and enzymatic hydrolysates. Heliyon. 4, 10 (Oct. 2018), e00877. PMid:30386828
View Article PubMed/NCBIAderinola, T.A., Fagbemi, T.N., Enujiugha, V.N., Alashi, A.M. and Aluko, R.E. 2018. In vitro antihypertensive and antioxidative properties of trypsin-derived Moringa oleifera seed globulin hydrolyzate and its membrane fractions. Food Science & Nutrition. (2018). PMid:30680166
View Article PubMed/NCBIAluko, R.E. 2004. The extraction and purification of proteins: an introduction. Proteins in Food Processing. Elsevier. 323-351.
View ArticleAluko, R.E. and Monu, E. 2003. Functional and bioactive properties of quinoa seed protein hydrolysates. Journal of Food Science. 68, 4 (2003), 1254-1258.
View ArticleAnhwange, B. a, Bjibola, V.O. and Oniye, S.J. 2004. Chemical studies of the seed of Moringa oleifera (Lam) and Detarium microcarpum (Guill abd Sperr). Journa of biological sciences.
Atawodi, S.E.., Atawodi, J.C.., Idakwo, G.A.., Pfundstein, B.., Haubner, R.., Wurtele, G.., Bartsch, H. and Owen, R.W. 2010. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. Journal of Medicinal Food. 13, (2010), 710-716. PMid:20521992
View Article PubMed/NCBIBenzie, I.. and Strain, J.. 1996. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Analytical biochemistry. 239, 1 (1996), 70-6. PMid:8660627
View Article PubMed/NCBIBerker, K.I., Güçlü, K., Tor, İ. and Apak, R. 2007. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta. 72, 3 (May 2007), 1157-1165. PMid:19071739
View Article PubMed/NCBIBidlingmeyer, B.A., Cohen, S.A. and Tarvin, T.L. 1984. Rapid analysis of amino acids using pre-column derivatization. Journal of Chromatography B: Biomedical Sciences and Applications. 336, 1 (1984), 93-104. 85133-6
View ArticleChedea, V.S., Braicu, C., Chirilă, F., Ogola, H.J.O., Pelmuş, R.Ş., Călin, L.G. and Socaciu, C. 2014. Antioxidant/Prooxidant and Antibacterial/Probacterial Effects of a Grape Seed Extract in Complex with Lipoxygenase. BioMed Research International. 2014, (2014), 1-9. PMid:25313359
View Article PubMed/NCBIDu, J. and Gebicki, J.M. 2004. Proteins are major initial cell targets of hydroxyl free radicals. International Journal of Biochemistry and Cell Biology. 36, April (2004), 2334-2343. PMid:15313477
View Article PubMed/NCBIGehrke, C., Wall, L., Absheer, J., Kaiser, F. and Zumwalt, R. 1985. Sample preparation for chromatography of amino acids: Acid hydrolysis of proteins. Journal - Association of Official Analytical Chemists. 68, 5 (1985), 811-821.
Girgih, A.T., Udenigwe, C.C. and Aluko, R.E. 2011. In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. JAOCS, Journal of the American Oil Chemists' Society. 88, 3 (2011), 381-389.
View ArticleGülçin, I., Elmastaş, M. and Aboul-Enein, H.Y. 2012. Antioxidant activity of clove oil - A powerful antioxidant source. Arabian Journal of Chemistry. 5, 4 (2012), 489-499.
View ArticleHuang, W., Majumder, K. and Wu, J. 2010. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chemistry. 123, 3 (2010), 635-641.
View ArticleKorhonen, H. and Pihlanto, A. 2003. Food-derived Bioactive Peptides - Opportunities for Designing Future Foods. (2003), 1297-1308. PMid:12769738
View Article PubMed/NCBILalas, S. and Tsaknis, J. 2002. Characterization of Moringa oleifera Seed Oil Variety "Periyakulam 1." Journal of Food Composition and Analysis. 15, 1 (2002), 65-77.
View ArticleLandry, J. and Delhaye, S. 1992. Simplified procedure for the determination of tryptophan of foods and feedstuffs from barytic hydrolysis. J agric food chem . 40, 5 (1992), 776-779.
View ArticleLi, Y., Jiang, B., Zhang, T., Mu, W. and Liu, J. 2008. Food Chemistry Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate ( CPH ). 106, (2008), 444-450.
View ArticleLuisi, G., Stefanucci, A., Zengin, G., Dimmito, M. and Mollica, A. 2018. Anti-Oxidant and Tyrosinase Inhibitory In Vitro Activity of Amino Acids and Small Peptides: New Hints for the Multifaceted Treatment of Neurologic and Metabolic Disfunctions. Antioxidants. 8, 1 (Dec. 2018), 7. PMid:30587771
View Article PubMed/NCBIMahomoodally, M.F., Zengin, G., Zheleva-Dimitrova, D., Mollica, A., Stefanucci, A., Sinan, K.I. and Aumeeruddy, M.Z. 2019. Metabolomics profiling, bio-pharmaceutical properties of Hypericum lanuginosum extracts by in vitro and in silico approaches. Industrial Crops and Products. 133, (Jul. 2019), 373-382.
View ArticleMalomo, S.A., He, R. and Aluko, R.E. 2014. Structural and functional properties of hemp seed protein products. Journal of Food Science. 79, 8 (2014), 1512-1521. PMid:25048774
View Article PubMed/NCBIMbah, B.O., Eme, P.E. and Paul, A.E. 2012. Effect of drying techniques on the proximate and other nutrient composition of Moringa oleifera leaves from two areas in Eastern Nigeria. Pakistan Journal of Nutrition. 11, 11 (2012), 1044-1048.
View ArticleMundi, S. and Aluko, R.E. 2014. Inhibitory Properties of Kidney Bean Protein Hydrolysate and its Membrane Fractions Against Renin , Angiotensin Converting Enzyme , and Free Radicals. Austin Journal of Nutrition and Food Sciences. 2, 1 (2014), 1008-1019.
Nam, K.A., You, S.G. and Kim, S.M. 2008. Molecular and Physical Characteristics of Squid (Todarodes pacificus) Skin Collagens and Biological Properties of Their Enzymatic Hydrolysates. Journal of Food Science. 73, 4 (May 2008), C249-C255. PMid:18460118
View Article PubMed/NCBIOgunsina, B.S., Radha, C. and Indrani, D. 2011. Quality characteristics of bread and cookies enriched with debittered Moringa oleifera seed flour. 62, March (2011), 185-194. PMid:21118057
View Article PubMed/NCBIPownall, T.L., Udenigwe, C.C. and Aluko, R.E. 2010. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) Enzymatic protein hydrolysate fractions. Journal of Agricultural and Food Chemistry. 58, 8 (2010), 4712-4718. PMid:20359226
View Article PubMed/NCBIRajapakse, N., Mendis, E., Byun, H. and Kim, S. 2005. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems B. 16, (2005), 562-569. PMid:16115545
View Article PubMed/NCBISaiga, A., Tanabe, S. and Nishimura, T. 2003. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. Journal of Agricultural and Food Chemistry. 51, 12 (2003), 3661-3667. PMid:12769542
View Article PubMed/NCBISamaranayaka, A.G.P. and Li-chan, E.C.Y. 2011. Food-derived peptidic antioxidants : A review of their production , assessment , and potential applications. Journal of Functional Foods. 3, 4 (2011), 229-254.
View ArticleSarmadi, B.H. and Ismail, A. 2010. Peptides Antioxidative peptides from food proteins : A review. Peptides. 31, 10 (2010), 1949-1956. PMid:20600423
View Article PubMed/NCBIStevens, G.C., Baiyeri, K.P. and Akinnnagbe, O. 2013. Ethno-medicinal and culinary uses of moringa oleifera Lam. in Nigeria. Journal of Medicinal Plants Research. 7, (2013), 799-804.
Tang, C., Wang, X. and Yang, X. 2009. Enzymatic hydrolysis of hemp ( Cannabis sativa L .) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chemistry. 114, 4 (2009), 1484-1490.
View ArticleTsukada, M., Nakashima, T., Kamachi, T. and Niwano, Y. 2016. Prooxidative Potential of Photo-Irradiated Aqueous Extracts of Grape Pomace, a Recyclable Resource from Winemaking Process. PLOS ONE. 11, 6 (Jun. 2016), e0158197. PMid:27341398
View Article PubMed/NCBIUdenigwe, C.C. and Aluko, R.E. 2012. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. Journal of Food Science. 77, 1 (2012). PMid:22260122
View Article PubMed/NCBIWang, J., Zhao, M., Zhao, Q. and Jiang, Y. 2007. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chemistry. 101, 4 (2007), 1658-1663.
View ArticleXie, Z., Huang, J., Xu, X. and Jin, Z. 2008. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chemistry. 111, (2008), 370-376. PMid:26047437
View Article PubMed/NCBIYen, G. and Hsieh, P. 1995. Antioxidative Activity and Scavenging Effects on Active Oxygen of Xylose-Lysine Maillard Reaction Products. Journal of the Science of Food and Agriculture. 67, 3 (1995), 415-420.
View ArticleYıldırım, A., Mavi, A. and Kara, A.A. 2001. Determination of Antioxidant and Antimicrobial Activities of Rumex crispus L . Extracts. Journal of Agricultural and Food Chemistry. 49, (2001), 4083-4089. PMid:11513714
View Article PubMed/NCBIYou, L., Zhao, M., Cui, C., Zhao, H. and Yang, B. 2009. Effect of degree of hydrolysis on the antioxidant activity of loach ( Misgurnus anguillicaudatus ) protein hydrolysates. Innovative Food Science and Emerging Technologies. 10, 2 (2009), 235-240.
View Article