Professor Wenhua Li,
Department of Cardiology, Affiliated Hospital of Xuzhou Medical University, 99 Huaihai West Road, Xuzhou, Jiangsu 221002, P.R. China
Email: xzwenhua0202@163.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 3
Page No: 687-695
Professor Wenhua Li,
Department of Cardiology, Affiliated Hospital of Xuzhou Medical University, 99 Huaihai West Road, Xuzhou, Jiangsu 221002, P.R. China
Email: xzwenhua0202@163.com
Dongmei Gao1 · Shun Yao1 · Yanhu Sun1 · Di Zheng2 · Quan Zhang2 · Wenhua Li12*
1 Institute of Cardiovascular Disease, Xuzhou Medical University
2 Department of Cardiology, Affiliated Hospital of Xuzhou Medical University
Dongmei Gao, Shun Yao and Yanhu Sun contributed equally
Jun-Ling Zhang(zhangjunling@irm-cams.ac.cn)
Kevin Nash(Knash@health.usf.edu)
Luciana Bordin(luciana.bordin@unipd.it)
Dongmei Gao, Astaxanthin attenuate iohexol-induced human proximal renal tubular epithelial cells injury via the ROS/NLRP3 inflammasome signal pathway (2019)SDRP Journal of Food Science & Technology 4(3)
BACKGROUND: To investigate the possible mechanism of renal protection of astaxanthin in iohexol-induced human proximal renal tubular epithelial cells injury.
METHODS: Human proximal renal tubular epithelial cells (HK-2) were randomly divided into six groups: blank control group (Control group); Dimethyl sulfoxide solvent control group (DMSO group); astaxanthin control group (AST group); Contrast media group (CM group); astaxanthin pretreatment group (AST+CM group); N-acetylcysteine pretreatment group (NAC+CM group).After the cells received different intervention for the indicated time,DAPI DNA fluorescence staining detected cells apoptosis; Annexin-V-FITC / PI dual-labeled flow cytometry was used to detect the apoptosis rate; the levels of Reactive oxygen species(ROS) was detected by Flow cytometry; the protein levels of NLRP3 and CASPASE1 were detected by Western Blotting; the levels of IL-1βand IL-18 was detected by ELISA.
RESULTS: The measurement results show a significant increase (P< 0.05) in the levels of ROS, NLRP3, CASPASE1, IL-1β, IL-18 and apoptosis rate in the CM group compared with the CON group. Compared with the CM group, a significant improvement in these unfavorable parameters was observed in AST+CM group and NAC+CM group. There was no significant difference in the above parameters between AST+CM group and NAC+CM group(P﹥0.05)
CONCLUSIONS: Astaxanthin can attenuate iohexol-induced human proximal renal tubular epithelial cells injury, and its possible mechanism is related to the inhibition of ROS production and down-regulation of NLRP3 inflammasome and its downstream apoptosis and inflammatory response.
Key words: Astaxanthin, CI-AKI, ROS, NLRP3 inflammasome, apoptosis, inflammatory
With the wide clinical application of coronary interventional techniques, especially in elderly patients with severe comorbidities, contrast-induced acute kidney injury (CI-AKI) is the third leading cause of hospital-acquired renal failure[1]. CI-AKI not only prolongs hospital stays, increases the financial burden on patients, but also increases long-term complications and mortality[2, 3]. However, there are currently no effective prevention and treatment measures in clinical practice. Therefore, early diagnosis, early prevention, and finding new drugs to treat CI-AKI are crucial. The occurrence of CI-AKI is the result of multiple factors. Studies had shown that the incidence of CI-AKI is mainly related to contrast media(CM) leading to renal microvascular hemodynamic changes, direct toxicity of CM to renal tubular cells, oxidative stress, apoptosis, inflammatory injury and tubular obstruction[4-6].
Astaxanthin (AST) is a xanthophyll carotenoid of predominantly marine origin, with potent antioxidant, anti-apoptosis and anti-inflammatory effects demonstrated in both experimental and human studies. Many studies have proven that astaxanthin has a preventive effect on various kidney diseases[7-10]. Oxidative stress, apoptosis and inflammatory injury are common pathophysiological features of CI-AKI, hence AST may have a potential therapeutic role in this condition. In the current study, we proposed to investigate the effect of Reactive oxygen species (ROS) inhibition on iohexol-induced renal tubular epithelial cell injury and the underlying mechanism through evaluating the NLRP-3 inflammasome-associated protein and the downstream inflammatory factors in HK-2. And further verify that the mechanism of astaxanthin ameliorate iohexol-induced renal tubular epithelial cell injury is through the ROS/NLRP3 pathway.
Materials and reagents
Astaxanthin (sigma, USA); Iohexol(Yangzijiang , China); N-Acetyl-L-cysteine(Aladdin, China); Fetal bovine serum(Sijiqing,China); DMEM/F12 medium(Hyclone, USA); penicillin/streptomycin(Beyotime, Shanghai, China); ROS Assay Kit (Beyotime, China); BCA Protein Kit, AnnexinV-FITC/PI Apoptosis Kit (Kaiji Biotechnology, China);DAPI (Invitrogen Biotechnology, USA); enhanced chemiluminescent detection kit (Shanghai Tianneng Technology Co., Ltd.); Radio Immunoprecipitation Assay (RIPA) Lysis Buffer (Beyotime, China); anti-NLRP3(CST, USA); anti-CASPASE1(Proteintech, USA); anti-β-actin(Proteintech, USA);IL-1β enzyme-linked immunosorbent assay (ELISA) kit and IL-18 enzyme-linked immunosorbent assay (ELISA) kit (ABclonal, China).
Cell culture and grouping
Human proximal renal tubular epithelial cells (HK-2) were purchased from the Chinese Type Culture Collection (CTCC, Shanghai, China), and were maintained in DMEM/F12 medium supplemented with 0.1% of a penicillin/streptomycin mixture and 10% fetal bovine serum. The cells were routinely grown in 10cm cell culture plates at 37°C in a humidified atmosphere with 5% carbon dioxide. When the cells grow to 70%~80%, continue to culture the cells for 24 h in serum-free medium,which will synchronize the cells to the stationary growth phase (G0/G1 phase). Then randomly divided the cells into 6 groups: blank control group (Control group); Dimethyl sulfoxide solvent control group (DMSO group): Add dimethyl sulfoxide to the medium to a concentration of 0.1%; astaxanthin control group (AST group): add AST to the medium to a concentration of 20μmol /L; Contrast media group (CM group): HK-2 cells were cultured in medium containing 100gI/L of iohexol for 24 h; astaxanthin pretreatment group (AST+CM group): HK-2 cells were pretreated with medium containing 5 mM AST for 18 h, and treated with medium containing 20μM AST and 100gI/L iohexol for 24h; N-acetylcysteine pretreatment group (NAC+CM group): HK-2 cells were pretreated with medium containing 5 mM N-acetylcysteine for 1 h, and treated with medium containing 5 mM N-acetylcysteine and 100gI/L iohexol for 24 h.
DAPI DNA fluorescent staining
Lightly wash HK-2 cells grown in 6-well culture plates with PBS solution 3 times, fixed cells in 4% paraformaldehyde for 15 min at room temperature, and then gently washed 3 times with PBS solution for 5 min. DAPI (4,6-diamidino-2-phenylindole, fluorescent DNA binding dye) was added to the cell culture plate for 5-10 min in the dark, and then washed 3 times with PBS solution for 5 min. The cells were observed on an Olympus fluorescence microscope with excitation light at 400 nm and emission at 455 nm.
Annexin V-Fluorescein Isothiocyanate Conjugated Propidium Iodide Staining
Gently rinsed the cells in the plates with 2 ml of PBS solution, and then removed the PBS solution. The cells were resuspended in the previous medium or pre-cooled 1×Binding Buffer to a cell concentration of approximately 1×106 cells/ml. Take 500 μl of cell suspension (5 × 105 cells) into a clean centrifuge tube, centrifuge at 1000g × 5min, and then remove the supernatant. The cells were washed once with 500μl of PBS, and the supernatant was removed after centrifugation. Then, 500 μl of 1×Binding Buffer was added to wash the cells again, and the supernatant was removed after centrifugation. After resuspending the cells with 100 μl of 1×Binding Buffer, 5 μl of Annexin V-APC was added to react at room temperature for 15 min in the dark. The cells were washed by adding 500 μl of 1× Binding Buffer, and the supernatant was removed after centrifugation. After resuspending the cells by adding 200 μl of 1× Binding Buffer, 5 μl of PI was added. The samples were analyzed by flow cytometry within 1 hour.
Western blotting
The cells were harvested using Radio Immunoprecipitation Assay (RIPA) Lysis Buffer. Cell lysates (10 µg) were electrophoresed on polyacrylamide gels and transferred to PVDF membranes. The membranes were blocked with TBS containing 3% bovine serum albumin and 0.1% Tween-20, and afterward incubated with the primary antibody and secondary antibodies and then analyzed using an enhanced chemiluminescent detection kit. Semi-quantitative Western-blot analysis was conducted through measuring the Optical Densitometry in three independent experiments using ImageJ software.
ELISA
The cells were digested with trypsin and collected in a sterile tube, then centrifuged for about 20 minutes (2000-3000 rpm). Collect the supernatant carefully. The supernatant was carefully collected and diluted with PBS to a cell concentration of about 106 cells/ml. The cells are destroyed by repeated freeze-thaw cycles and the intracellular components are released. Centrifuge for about 20 minutes (2000-3000 rpm). Collect the supernatant carefully. The levels of IL-1β and IL-18 were detected with ELISA kits according to the manufacturer’s instructions. The absorbance was measured by a microplate reader.
Flow cytometry for detection of intracellular ROS levels
The cells in the logarithmic growth phase were digested, centrifuged, counted on a cell counting plate, and the cell seeding density was 4×105 cells/ml. The cells were seeded in a 6-well plate and cultured for 24 hours until the cells were attached. The cells were treated with the corresponding drugs according to the group: the negative control group had no special treatment; the positive control group was added with Rosoup 1 μl +1 ml DCFH-DA; after the treatment time of each group, except for the negative control group, each experimental group was added 1ml fluorescent probe DCFH-DA(10 μmol/ L). After culturing the cells for 30 min at 37 ° C in a 5% CO 2 incubator, the medium was discarded, and washed with serum-free medium for 3 times × 5 min to completely remove DCFH-DA which did not enter the cells. The cells were digested with trypsin and collected, and the cells were placed in a flow cytometer to measure ROS levels. The excitation light of the flow cytometer was set to 488 nm, and the emitted light was set to 525 nm.
Statistical analysis
Statistical analysis was performed using Graphpad Prism 5.0 software. All data were expressed as mean ± standard deviation (`x±s). The rate was compared using the χ2 test, and the t-test was used for comparison between the two groups. The comparison between the data of multiple groups was analyzed by one-way ANOVA. P<0.05 indicates that the difference was statistically significant.
Detection of Proliferation Activity by CCK8
The results of CCK-8 showed (Figure 1): Compared with the Control group, there was no significant difference in the DMSO group and the AST group; the proliferation activity of the CM group was significantly decreased(P<0.05). The proliferation activity in the AST+CM group and the NAC+CM group were significantly higher(P<0.05) than that in CM group. There was no significant difference in cell proliferation activity between the AST+CM group and the NAC+CM group.
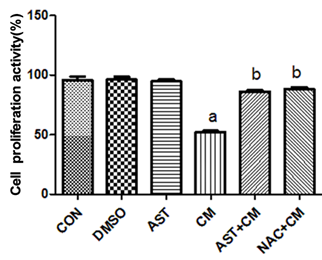
Figure 1. Comparison of proliferation activity of HK-2 cells in each group(n=5) aP<0.05 versus the Control group; bP<0.05 versus the CM group
Detection of Expression Levels of NLRP3 and CASPASE1 by Western Blotting
Western blotting showed that there was no significant difference between DMSO, and AST group compared with Control group; NLRP3 and CASPASE1 protein expression significant increase in CM group (P<0.05). The expression of NLRP3 and CASPASE1 protein was significantly decreased after AST pretreatment (P<0.05). After administration of ROS inhibitor NAC, the content of NLRP3 and CASPASE1 protein was also significantly decreased (P<0.05). (Figure 2).
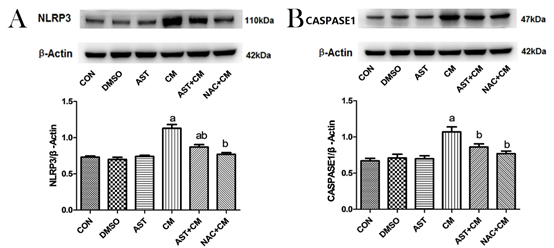
Figure 2. Protein expression of NLRP3 and CASPASE1 in HK-2 cells of each group(n=3)
(A)Representative western blot analysis of the protein expression of NLRP3. (B) Representative western blot analysis of the protein expression of CASPASE1. β-Actin was used as the loading control. Data were normalized to the expression level of β-Actin. aP<0.05 versus the Control group; bP<0.05 versus the CM group
DAPI fluorescent staining:
In this experiment, the morphology of the nucleus was observed by DAPI DNA fluorescent staining. DAPI can be combined with the A-T base of double-stranded DNA. After DAPI staining, the nucleus will emit pale blue-white fluorescence. The nucleus of normal cells is approximately circular, with clear edges, uniform staining and light staining. Early apoptotic cells showed nuclear pyknosis and nuclear staining deepened; late apoptotic cells showed a nuclear fragmentation into circular bodies of different sizes, which were surrounded by cell membranes, i.e. apoptotic bodies, and the DAPI staining fluorescence of the nucleus was stronger. The experimental results are shown in Figure 3A: Control group, DMSO group and AST group, the nuclear staining was uniform, no apoptotic cells were observed. The cells in the CM group were inferior to the control group. The nucleus was pyknotic and the nucleus was deeply stained. The condensed nucleus was highlighted, and some cells showed nuclear lysis, which was apoptotic cells. Compared with the CM group, the nucleus pyknosis, nuclear deep staining, and apoptotic cells were reduced after pretreatment with ROS inhibitor NAC. Similar to the results of the NAC+CM group, apoptotic cells were significantly reduced in the AST-treated group.
Detection of Apoptosis rate by Annexin V /PI
Compared with the Control group, there was no significant difference in the DMSO group and the AST group; while, the apoptosis rate of the CM group was significantly increased (P<0.05). The apoptosis rates of the AST+CM group and NAC+CM group were significantly lower than that of CM group (P<0.05). There was no significant difference between AST group and NAC group (P>0.05). (Figure 3B)
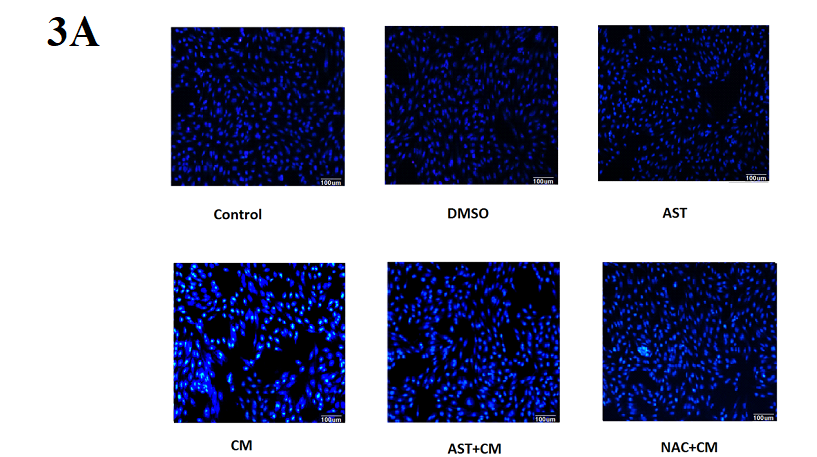
Figure 3A. DAPI fluorescence staining of HK-2 cells in each group (×200)
Control group, DMSO group and AST group, the nuclear staining was uniform, no apoptotic cells were observed. The cells in the CM group were inferior to the control group. The nucleus was pyknotic and the nucleus was deeply stained. The condensed nucleus was highlighted, and some cells showed nuclear lysis, which was apoptotic cells. Compared with the CM group, the nucleus pyknosis, nuclear deep staining, and apoptotic cells were reduced after pretreatment with ROS inhibitor NAC. Similar to the results of the NAC+CM group, apoptotic cells were significantly reduced in the AST-treated group.
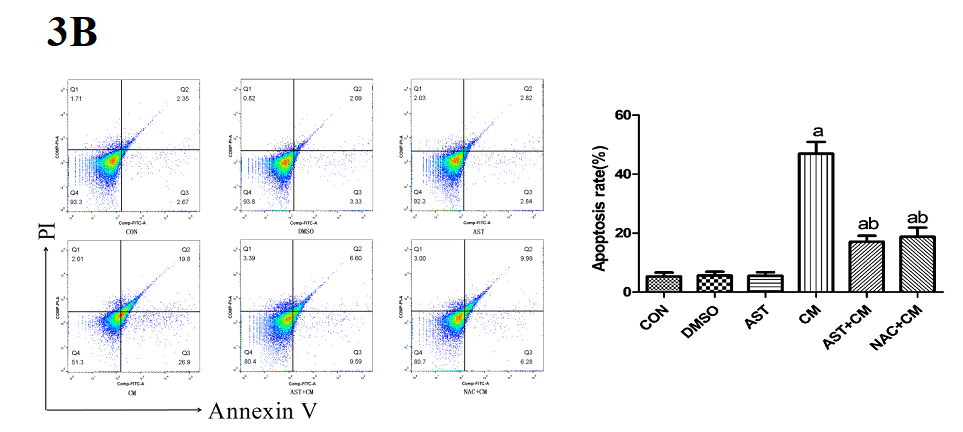
Figure 3B. Apoptosis rate detected by flow cytometry after Annexin V /PI staining(n=3)
The apoptosis rate is the sum of Q2 and Q3. Q2, Annexin V+/PI+ represents the rate of late apoptotic cells; Q3, Annexin V+/PI- represents the rate of early apoptotic cells; Q4, Annexin V- /PI- represents the rate of living cells. FITC, fluorescein isothiocyanate; PI, propidium iodide. aP<0.05 versus the Control group; bP<0.05 versus the CM group
Detection of Expression Levels of IL-1β and IL-18 by ELISA
Compared with the Control group, there was no significant difference in the DMSO and AST groups; while, the expression levels of IL-1β and IL-18 in the CM group were significantly increased (P<0.05). The expression levels of IL-1β and IL-18 in the AST+CM group and the NAC+CM group was significantly decreased (P<0.05). However, there was no significant difference between the AST+CM group and the NAC+CM group. (Figure 4).
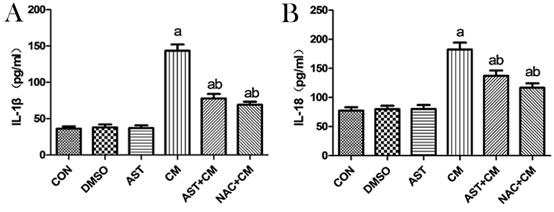
Figure 4. Expression levels of IL-1β and IL-18 in HK-2 cells of each group(n=3)
The expression levels of IL-1β and IL-18 was detected by ELLISA Kits. Results are presented relative to the value of the CON group and the CM group samples. aP<0.05 versus the Control group; bP<0.05 versus the CM group.
Detection of Expression Levels of ROS by Flow cytometry (FCM)
ROS, including all unstable products produced by O2 metabolism, such as super active superoxide anion • O2-, HO• and non-free radical molecules H2O2. They are produced by aerobic metabolism, and the production of ROS increases when oxidative stress occurs in the body. Compared with the Control group, there was no significant difference in the DMSO group and the AST group; while, the ROS content in the CM group was significantly increased (P<0.05). The ROS level of AST+CM group and NAC+CM group was significantly lower than that of CM group (P<0.05). The results show that both AST pretreatment and NAC pretreatment can reduce the production level of ROS in HK-2 cells. (Figure 5)
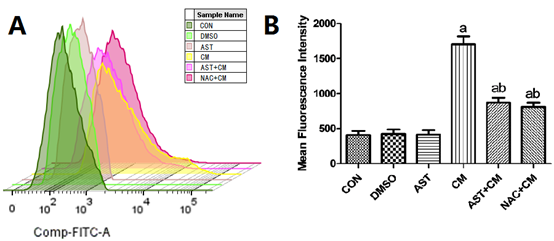
Figure 5. Reactive oxygen species levels in each group of HK-2 cells(n=3)
(A) The flow histogram of ROS was detected by Flow cytometry; ( B) Reactive oxygen species levels in each group of HK-2 cells. Results are presented relative to the value of the CON group and the CM group samples. aP<0.05 versus the Control group; bP<0.05 versus the CM group. FITC, fluorescein isothiocyanate.
Contrast media, might giving rise to renal hypoxic-ischemic, oxidative stress or direct cytotoxicity of itself and so on, has been supposed to be a causative and aggravating factor of progressive AKI[11-13]. The occurrence of CI-AKI increases the medical burden of patients. Some serious patients will progress to chronic renal failure, and some even need renal replacement therapy, which seriously threatens the prognosis of patients. Hydration therapy is currently recognized as an effective measure to prevent CI-AKI, and is a simple, effective and economical method for preventing CI-AKI[14]. However, for patients with advanced age, hypertension, and heart and renal dysfunction, the use of hydration therapy is limited. In addition, hydration therapy combined with drug intervention can further reduce the incidence of CI-AKI and improve the long-term prognosis of patients[15]. Therefore, the research of drugs has gradually become a research hotspot of CI-AKI prevention. The results of this study showed that AST exerts a renal protective effect on CI-AKI by increasing cell proliferation activity, alleviating apoptosis, and releasing inflammatory factors such as IL-1β and IL-18.
Recent studies have shown that NLRP3 inflammasome are closely related to the innate immune response of the body. Multiple pathogen-associated molecular patterns and risk-related molecular patterns can activate inflammasome, cause the production of pro-inflammatory cytokines such as IL-1β and provoke apoptosis [16, 17]. Emerging studies have shown that NLRP3 inflammasome plays an important role in the development of CI-AKI in vivo and in vitro studies, and mediates downstream apoptosis and inflammatory responses[18, 19].It is well known that the mechanism of CI-AKI is closely related to oxidative stress, apoptosis and inflammatory response[20, 21]. It has been reported that oxidative stress plays an important role in CI-AKI[22, 23]. Increased production of ROS can induce inflammatory responses by increasing the direct or indirect expression of pro-inflammatory cytokines and chemokines. Reactive oxygen species (ROS) is currently recognized as one of the key molecules that activate the NLRP3 inflammatory complex[24-26]. However, whether NLRP3 inflammatory body-associated protein and its downstream inflammatory factors are regulated by ROS in CI-AKI or not is unclear yet. Our in vitro cells-based study showed that ROS expression is up-regulated and NLRP3 inflammasome was associated with cell apoptosis and inflammatory response in CI-AKI. Combined with the results after inhibiting the expression of ROS by NAC, the inhibitor of ROS, the study confirmed that ROS can activate NLRP3 inflammasome and its downstream apoptosis and inflammatory response.
Astaxanthin (AST) is a naturally occurring red carotenoid derivative widely found in many animals and plants, especially marine organisms such as microalgae, salmon, shrimp, squid, crab, etc. The special structure of AST imparts superior antioxidant activity to it, which is hundreds of times more reductive than vitamin E. AST is the strongest antioxidant found in nature to date. Recent researches demonstrated that the antioxidant activity of AST plays a protective role in acute kidney injury induced by various nephrotoxic drugs, such as such as colistin, cisplatin and ferric nitrilotriacetate[27-29]. Studies have confirmed that astaxanthin can alleviate acute kidney injury caused by iohexol, and in the process, the decline of oxidative stress index and the regulation of apoptosis and inflammation-related proteins are observed[30, 31]. However, the connection between AST and the NLRP3 inflammasome in CI-AKI hasn't been investigated yet. Consistent with these studies about inhibition of NLRP3 inflammasome, our results showed that CM induced excess ROS generation, activated NLRP3 inflammasome in HK2 cells. AST suppressed excess ROS generation induced by CM, and significantly decreased the elevated activation of NLRP3 inflammasome as well as the cleaved CASPASE1 and IL-1β expressions induced by CM in HK2 cells. The experimental results confirmed that pretreatment with AST can inhibit the overexpression of ROS in HK2 cells, thereby inhibiting the NLRP3 inflammasome and its downstream apoptosis and inflammatory response. The results were not statistically different from those obtained after NAC pretreatment. Therefore, we hypothesized that the protective effect of AST on iohexol-induced human proximal renal tubular epithelial cells injury might be through inhibition of ROS/NLRP3 inflammatory signaling pathway.
In conclusion, our study demonstrated that AST could attenuate the acute kidney injury induced by CM. Furthermore, the underlying mechanisms of the renal protective effects of AST were preliminarily verified through suppressing the ROS generation, alleviating the activation of NLRP3 inflammasome and its downstream apoptosis and inflammatory response. Taken together, these results suggested that AST attenuate iohexol-induced human proximal renal tubular epithelial cells injury via the ROS/NLRP3 inflammasome signal pathway.
Funding
This study was supported by a grant from the Six talent peaks project in Jiangsu Province(2014-YY-007)
Author's contributions
Dongmei Gao, Shun Yao and Yanhu Sun involved in conception and design, or acquisition of data, or analysis and interpretation of data, the contributions of them are equal. Di Zheng and Quan Zhang involved in acquisition of data, or analysis and interpretation. Dongmei Gao involved in drafting the manuscript. Wenhua Li revised and approved the manuscript.
Nash K, Hafeez A and Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002; 39: 930-936. PMid:11979336
View Article PubMed/NCBISolomon R. Contrast-induced acute kidney injury (CIAKI). Radiol Clin North Am 2009; 47: 783-788, v. PMid:19744593
View Article PubMed/NCBIWeisbord SD and Palevsky PM. Contrast-induced acute kidney injury: short- and long-term implications. Semin Nephrol 2011; 31: 300-309. PMid:21784279
View Article PubMed/NCBISeeliger E, Sendeski M, Rihal CS and Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 2012; 33: 2007-2015. PMid:22267241
View Article PubMed/NCBIFahling M, Seeliger E, Patzak A and Persson PB. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol 2017; 13: 169-180. PMid:28138128
View Article PubMed/NCBIPflueger A, Abramowitz D and Calvin AD. Role of oxidative stress in contrast-induced acute kidney injury in diabetes mellitus. Med Sci Monit 2009; 15: Ra125-136.
Augusti PR, Conterato GM, Somacal S, Sobieski R, Spohr PR, Torres JV, Charao MF, Moro AM, Rocha MP, Garcia SC and Emanuelli T. Effect of astaxanthin on kidney function impairment and oxidative stress induced by mercuric chloride in rats. Food Chem Toxicol 2008; 46: 212-219. PMid:17881112
View Article PubMed/NCBIWang X, Zhao H, Shao Y, Wang P, Wei Y, Zhang W, Jiang J, Chen Y and Zhang Z. Nephroprotective effect of astaxanthin against trivalent inorganic arsenic-induced renal injury in wistar rats. Nutr Res Pract 2014; 8: 46-53. PMid:24611105 PMCid:PMC3944156
View Article PubMed/NCBIGuo SX, Zhou HL, Huang CL, You CG, Fang Q, Wu P, Wang XG and Han CM. Astaxanthin attenuates early acute kidney injury following severe burns in rats by ameliorating oxidative stress and mitochondrial-related apoptosis. Mar Drugs 2015; 13: 2105-2123. PMid:25871290
View Article PubMed/NCBIQiu X, Fu K, Zhao X, Zhang Y, Yuan Y, Zhang S, Gu X and Guo H. Protective effects of astaxanthin against ischemia/reperfusion induced renal injury in mice. J Transl Med 2015; 13: 28. PMid:25623758
View Article PubMed/NCBIPersson PB and Tepel M. Contrast medium-induced nephropathy: the pathophysiology. Kidney Int Suppl 2006; S8-10. PMid:16612403
View Article PubMed/NCBINaziroglu M, Yoldas N, Uzgur EN and Kayan M. Role of contrast media on oxidative stress, Ca(2+) signaling and apoptosis in kidney. J Membr Biol 2013; 246: 91-100. PMid:23132012
View Article PubMed/NCBIPerrin T, Descombes E and Cook S. Contrast-induced nephropathy in invasive cardiology. Swiss Med Wkly 2012; 142: w13608.
View ArticleOzgur T, Tutanc M, Zararsiz I, Motor S, Ozturk OH, Yaldiz M and Kurtgoz OY. The protective effect of ebselen on radiocontrast-induced nephrotoxicity. Ren Fail 2012; 34: 991-997. PMid:22880804
View Article PubMed/NCBIBriguori C and Marenzi G. Contrast-induced nephropathy: pharmacological prophylaxis. Kidney Int Suppl 2006; S30-38. PMid:16612399
View Article PubMed/NCBIWeigt SS, Palchevskiy V and Belperio JA. Inflammasomes and IL-1 biology in the pathogenesis of allograft dysfunction. J Clin Invest 2017; 127: 2022-2029. PMid:28569730
View Article PubMed/NCBITricarico PM, Epate A, Celsi F and Crovella S. Alendronate treatment induces IL-1B expression and apoptosis in glioblastoma cell line. Inflammopharmacology 2018; 26: 285-290. PMid:28646347
View Article PubMed/NCBIShen J, Wang L, Jiang N, Mou S, Zhang M, Gu L, Shao X, Wang Q, Qi C, Li S, Wang W, Che X and Ni Z. NLRP3 inflammasome mediates contrast media-induced acute kidney injury by regulating cell apoptosis. Sci Rep 2016; 6: 34682. PMid:27721494
View Article PubMed/NCBITan X, Zheng X, Huang Z, Lin J, Xie C and Lin Y. Involvement of S100A8/A9-TLR4-NLRP3 Inflammasome Pathway in Contrast-Induced Acute Kidney Injury. Cell Physiol Biochem 2017; 43: 209-222. PMid:28854431
View Article PubMed/NCBIJeong BY, Lee HY, Park CG, Kang J, Yu SL, Choi DR, Han SY, Park MH, Cho S, Lee SY, Hwang WM, Yun SR, Ryu HM, Oh EJ, Park SH, Kim YL and Yoon SH. Oxidative stress caused by activation of NADPH oxidase 4 promotes contrast-induced acute kidney injury. PLoS One 2018; 13: e0191034. PMid:29329317
View Article PubMed/NCBIZhang Q, Liu X, Li N, Zhang J, Yang J and Bu P. Sirtuin 3 deficiency aggravates contrast-induced acute kidney injury. J Transl Med 2018; 16: 313. PMid:30445987
View Article PubMed/NCBIHeyman SN, Rosen S, Khamaisi M, Idee JM and Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 2010; 45: 188-195. PMid:20195159
View Article PubMed/NCBIPisani A, Riccio E, Andreucci M, Faga T, Ashour M, Di Nuzzi A, Mancini A and Sabbatini M. Role of reactive oxygen species in pathogenesis of radiocontrast-induced nephropathy. Biomed Res Int 2013; 2013: 868321. PMid:24459673
View Article PubMed/NCBIGroslambert M and Py BF. [Regulation of the NLRP3 inflammasome]. Med Sci (Paris) 2018; 34: 47-53. PMid:29384096
View Article PubMed/NCBIDing T, Wang S, Zhang X, Zai W, Fan J, Chen W, Bian Q, Luan J, Shen Y, Zhang Y, Ju D and Mei X. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine 2018; 41: 45-53. PMid:29519318
View Article PubMed/NCBILatz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol 2010; 22: 28-33. PMid:20060699
View Article PubMed/NCBIGhlissi Z, Hakim A, Sila A, Mnif H, Zeghal K, Rebai T, Bougatef A and Sahnoun Z. Evaluation of efficacy of natural astaxanthin and vitamin E in prevention of colistin-induced nephrotoxicity in the rat model. Environ Toxicol Pharmacol 2014; 37: 960-966. PMid:24709323
View Article PubMed/NCBIAkca G, Eren H, Tumkaya L, Mercantepe T, Horsanali MO, Deveci E, Dil E and Yilmaz A. The protective effect of astaxanthin against cisplatin-induced nephrotoxicity in rats. Biomed Pharmacother 2018; 100: 575-582. PMid:29494988
View Article PubMed/NCBIOkazaki Y, Okada S and Toyokuni S. Astaxanthin ameliorates ferric nitrilotriacetate-induced renal oxidative injury in rats. J Clin Biochem Nutr 2017; 61: 18-24. PMid:28751805
View Article PubMed/NCBIGao D, Wang H, Xu Y, Zheng D, Zhang Q and Li W. Protective effect of astaxanthin against contrast-induced acute kidney injury via SIRT1-p53 pathway in rats. Int Urol Nephrol 2018; PMid:30456546
View Article PubMed/NCBILiu N, Chen J, Gao D, Li W and Zheng D. Astaxanthin attenuates contrast agent-induced acute kidney injury in vitro and in vivo via the regulation of SIRT1/FOXO3a expression. Int Urol Nephrol 2018; PMid:29368247
View Article PubMed/NCBI