Prof. Yumin Li
Phone: +86 (0931)5190-900
Email: liym@lzu.edu.cn
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 3
Page No: 659-669
Prof. Yumin Li
Phone: +86 (0931)5190-900
Email: liym@lzu.edu.cn
Xiaohui Wang1,2, Yumin Li2,3
1 Nuclear Medicine Department, Lanzhou University Second Hospital, Lanzhou, 730000, China
2 Key Laboratory of Digestive System Tumors of Gansu Province, Lanzhou University Second Hospital, Lanzhou 730000, China
3Department of General Surgery, Lanzhou University Second Hospital, Lanzhou 730000, China
Xiaowu Xu(xuxiaowu@fudanpci.org)
Yumin Li, Xiaohui Wang, PET Imaging in Pancreatic Cancer(2019)SDRP Journal of Food Science & Technology 4(3)
Early accurate detection of pancreatic cancer is still a challenge for current medicine. Compared to conventional anatomical imaging techniques, PET can provide information on tumor function, and PET/CT is increasingly used in detecting and staging of cancer as single "one stop shop" method. In this review, we summarize current PET molecular imaging tracers or probes for pancreatic cancer detection, and a perspective of the future trend of pancreatic cancer target-specific probes in the clinic is also provided.
Keywords: Pancreatic cancer, Pancreatic ductal adenocarcinoma, positron emission tomography (PET)
Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic cancer, which is currently considered to be the third leading cause of cancer-related deaths in the United States[1]. Only 10 to 20% of patients with pancreatic cancer are candidates for resection and hence have any potential for cure, and the majority of patients present in late stages [2]. Conventional diagnostic imaging, such as ultrasonography and endoscopic ultrasound (EUS), Computed tomography (CT), MRI (Magnetic Resonance Imaging) are available structual imaging techniques for the diagnosis, staging, and management of pancreatic neoplasms. Trans-abdominal ultrasound is a first-line screening modality for evaluating patients with suspicious pancreatic disease, because of its advantages include wide availability, low cost, and lack of radiation. However, it is sometimes difficult to evaluate the entire pancreas because of gas-fat interferences and the diagnostic ability greatly depends on the operator’s experience. EUS combines ultrasound with endoscopy, overcomes those limitations and obtains a higher resolution imaging of the pancreas and adjacent structures. It is also possible to obtain tissue specimen for histologic diagnosis using EUS-guided fine needle aspiration. However, it also has several limitations such as lack of widespread availability, a small field of view, and the need for patient sedation. Enhanced CT with iodinated contrast medium is now routinely performed for the diagnosis of suspicious pancreatic lesions, especially for assessment of resectability, assessment of vascular invasion with good spatial and temporal resolution. MRI is superior to CT in the evaluation of soft tissue and static fluid. A variety of techniques are used for further identification and characterization of pancreatic diseases:dynamic studies following gadolinium injection; magnetic resonance cholangio-pancreatography (MRCP); and diffusion-weighted imaging (DWI). MRCP allows the non-invasive delineation of the pancreatic duct and biliary tract. This technique will probably replace invasive endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis of small pancreatic masses , although its disadvantage is that it does not permit tissue sampling.However,these used structual imaging modalities are not specific in disease diagnosis, then distinguishing pancreatic adenocarcinoma from nonmalignant masses remains a challenge [3, 4], especially for lesions smaller than 2 cm and cause an inconspicuous border deformity of the pancreas[5]. Meanwhile, chronic pancreatitis is notoriously difficult to diagnose, no applicable blood test currently used for chronic pancreatitis, and consequently this disease is diagnosed mainly through conventional insensitive imaging techniques. Hence, distinguish chronic pancreatitis from pancreatic cancer is still a difficulty, there is no established method for early detection of pancreatic cancer [1, 6].
Contrast to conventional anatomical imaging techniques, molecular imaging modalities such as positron emission tomography (PET) can provide information on tumor function, it is a nuclear imaging technique used to visualize, characterize, and measure biological processes at the cellular, subcellular, and molecular level in living subjects non-invasively[7]. In combination with probes or tracers that bind to and enable detection of disease-specific molecules[4]. Currently, hardware fusion PET/CT imaging is the general trend. Hardware fusion PET/CT not only increases diagnostic accuracy, but also significantly decreases the time required for attenuation correction. Numerous targeting moieties have been employed as vehicles of PET probes, including small molecules, peptides, protein, antibody and its fragments, as well as nanoparticles. This review summarizes current PET molecular imaging tracers or probes for pancreatic cancer detection and provides an overview of the current status and trends in the development of pancreatic cancer target-specific probes.
2-deoxy-2-[18F]-fluoro-d-glucose (18F-FDG) imaging of glucose metabolism
To date, the glucose analogue 18F-FDG PET has been the most commonly used radiotracer wordwide. At present, it is most applied for staging, planning treatment, predicting prognosis, monitoring the response to therapy, evaluating recurrences as well. As glucose metabolism changes in tissue usually predate any structural changes of the pancreas, 18F-FDG may be more sensitive for detecting early malignancies. Rijkers et al.[8] performed a meta-analysis, in which thirty-five studies were included. Pooled estimates for 18F-FDG PET/CT were: sensitivity 90%, specificity 76%, PPV 89%, NPV 78% and accuracy 86%, respectively. The pooled sensitivity and specificity for 18F-FDG PET/CT to differentiate between pancreatic cancer and chronic pancreatitis were 90% and 84%, respectively. It concluded that 18F-FDG PET/CT showed no superiority to the current primary diagnostic tools in diagnosing pancreatic cancer. First, 18F-FDG is a non-specific imaging tracer, increased glucose metabolism at inflammatory lesions is the main source of false-positive. Second, due to the presence of pancreatic cancer or underlying disease, a high percentage of these patients are diabetic, and elevated plasma glucose levels will cause a high rate of the false negative. Therefore, both false positive and false negative are common for 18F-FDG in pancreatic cancer diagnosis.
[18F]-fluoro-3-deoxy-3-fluorothymidine(18F-FLT) imaging of cellular proliferation
Thymidine is a native nucleoside, which is exclusively incorporated into the cellular DNA. 18F-FLT, an analogue of thymidine, can be performed for clinical evaluation and quantification of proliferative activity and tumor invasiveness. There many clinical researches evaluated the potential value of 18F-FLT PET/CT for imaging pancreatic adenocarcinoma [2, 9, 10]. Quon et al.[9] compared 18F-FLT and 18F-FDG PET/CT scan in five patients. On 18F-FLT PET/CT, the primary pancreatic adenocarcinoma was detected in 40% patients from background activity. By contrast, 18F-FDG uptake was higher in each patient and primary cancer could be detected in 100% patients. Clinical studies performed so far have not shown a distinct advantage for 18F-FLT over 18F-FDG. Overall, tumor 18F-FLT uptake is lower than 18F-FDG uptake in most cancers, reflecting the higher sensitivity of 18F-FDG.
However, how about 18F-FDG and 18F-FLT imaging in the context of infection and inflammation? Van et al.[11] compared 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. They discovered in 18F-FDG PET images, both tumor and inflammation were visible, but 18F-FLT PET showed only the tumor. Thus, it was hypothesized that 18F-FLT has a higher tumor specificity in rodent model. However, its potential in differentiation between tumor and inflammation has not yet been evaluated in humans, further studies may be required.
Imaging with antibodies-based probes
Yet most novel imaging probes are hindered by suboptimal tumor accumulation, to overcome these limitations, researchers have explored numerous antibodies for PET imaging purposes [12-15]. Antibody-based probes have advantages of antigen-specific, high binding affinity and absolute tumor uptake.
1) 64Cu-Labeled MAb159
The glucose-regulated protein78 (GRP78) receptor is overexpressed on the surface of tumor cells, and it is capable of serving as a receptor or target of anti-cancer drugs[16]. Wang et al.[15] developed a 64Cu-labeled monoclonal antibody, MAb159 for PET imaging of tumor GRP78 expression. In BXPC3 xenografts, 64Cu-DOTA-MAb159 demonstrated prominent tumor accumulation (15.4 ± 2.6, and 18.3±1.0%ID/g at 17h, and 48 after injection, respectively). On contrary, 64Cu-DOTA–human IgG had much lower tumor accumulation. It was demonstrated that GRP78 can serve as a valid target for pancreatic cancer imaging and may also have important applications in other types of cancer with high expression of GRP78.
2) Zirconium-89 (89Zr)-labeled anti-IGF-1R antibody
Insulin-like growth factor-1 receptor (IGF-1R) plays an important role in cancer tumorigenesis. England et al.[17] reported the development of an 89Zr labeled anti-IGF-1R antibody (89Zr-Df-1A2G11) for PET imaging of pancreatic cancer. Serial PET imaging was performed at different time points after 89Zr-Df-1A2G11 was injected into MIA PaCa-2, BxPC-3, and AsPC-1 tumor bearing mice. The highest accumulation of 89Zr-Df-1A2G11 was found in the MIA PaCa-2-derived tumor model at 12 h postinjection (7.28 ± 1.36%ID/g). This study provides initial evidence that 89Zr-labeled IGF-1R-targeted antibody may be employed for imaging pancreatic cancer.
3)64Cu labeled ALT-836
The expression of tissue factor (TF) is upregulated in many solid tumors. Weibo Cai’s group ever first successfully developed TF targeted imaging probe for pancreatic cancer detection [18]. ALT-836 is a TF monoclonal antibody that can bind to TF with subnanomolar affinity. 64Cu-NOTA-ALT-836 is capable of binding TF, and it revealed that the uptake of the tracer was 16.5±2.6%ID/g in BXPC-3 pancreatic cancer models with high TF expression at 48 h after injection (Fig.1). Furthermore, the biodistribution data were consistent with the PET findings.
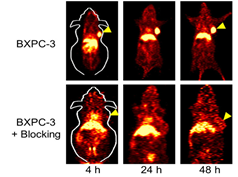
Fig.1 64Cu labeled ALT-836 imaging of TF and its block study[18].
4) 124I-labeled anti-CA19-9 diabody
Due to a long serum half-life (10–20d), intact antibodies are poor imaging agents. Slow tumor accumulation and high background signal are the consequence of inconspicuous contrast between the tumor and surrounding blood. Smaller engineered antibody fragments undoubtedly overcome this problem. The diabody (55 kDa) is the smallest antibody fragment (serum half-life:2-4h). Girgis et al.[19] successfully engineered a functional diabody against CA19-9, and an isotope iodine-124 (124I) was labeled to it. It was proved that 124I-anti-CA19-9 diabody demonstrated high contrast antigen specific in pancreas xenograft imaging. The average tumor/blood ratio of nude mice carrying CA19-9 positive and negative models with the 124I-labeled anti-CA19-9 diabody was 5.0 and 2.0, respectively, and the average tumor ratio of positive/negative was 11 and 6, respectively.
Peptides based tracers for imaging
As compared to antibodies, low-molecular-weight peptides have their distinctive advantages: short blood lifetime; non-immunogenic; relatively inexpensive to synthesize; easy to modify [7, 20]. Consequently, numerous peptide-based agents have been developed to the specific molecular targets in preclinical and clinical studies (Table 1).
1. Targeting αvβ6 integrin
Integrin alphavbeta6 (αvβ6) is one of cell surface receptors low expressed in the mature tissue, but significantly up-regulated in PDAC, which was considered to be a promising target for diagnostic imaging and therapy[21, 22].
i) Peptide NAVPNLRGDLQVLAQKVART (A20FMDV2) was derived from foot-and-mouth disease virus with 20 amino acids, which presented a potent inhibition of αvβ6 ( IC50 3 nmol/L) was identified by the Hausner group[20]. A20FMDV2 was radiolabeled by using 4-[18F] fluorobenzoic acid, and it was a first-generation radiotracer for targeting αvβ6 in vivo. It appeared rapid uptake (<30 min) and selective long retention (>5 h) of radioactivity in the αvβ6-positive tumor, the ratio of tumor-to-background was steady over time, and the tracer with fast renal elimination. Several mice also underwent [18F] FDG Micro-PET scan 1 h after injection, and there was no difference in [18F] FDG uptake between positive and negative mice models. [18F]FBA-PEG(28)-A20FMDV2 was an improved αvβ6 imaging agent based on the former study(Fig.2)[23]. The modified agent maintained a higher affinity for αvβ6 and showed significantly improved αvβ6-dependent binding. In BxPC-3 tumors, the modified tracer showed retention of 12-fold greater than retention of the non-PEGylated [18F] FBA-A20FMDV2. At 4 hours, tumor-to-pancreas and tumor-to-blood biodistribution ratios achieved >23:1 and >47:1, respectively. Significantly, [18F] FBA-PEG(28)-A20FMDV2 was superior to [18F]FDG in imaging the BxPC-3 tumors, and it has potential in clinics for αvβ6-specific tumor imaging.
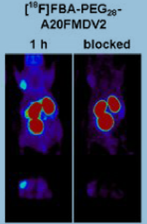
Fig.2 [18F]FBA-PEG(28)-A20FMDV2 microPET imaging of αvβ6 in pancreatic cancer xenografts and block study[23].
ii)Hackel Group[24] synthesized peptides R01 and S02 (Figs.3), and were conjugated with 18F-fluorobenzoate. After injection for both peptides, the tumor was clearly visualized as early as 0.5 h (Figs.4). 18F-fluorobenzoate-R01 presents greater tumor uptake than 18F-fluorobenzoate-S02, and both have comparable tumor-to-muscle ratios: 3.1±1.0 and 2.9±0.4 at 0.5 and 1 h, respectively. Imagings were also performed with integrin avb6–negative xenografted mice. Both exhibits significantly less uptake than BxPC3 xenografts (Figs.4).
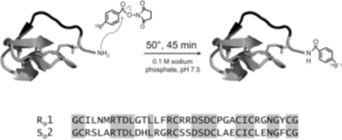
Fig. 3 R01 and S02 are cystine knot peptides. N-terminal amine was coupled with 18F-SFB. Peptide sequences are presented, conserved residues were highlighted[24].
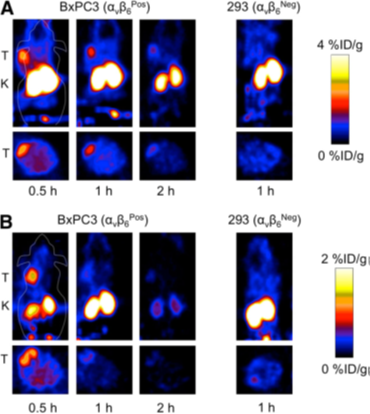
Fig. 4 Micro-PET imaging. 18F-fluorobenzoate-R01 (A) or 18F-fluorobenzoate-S02 (B) were injected into nude mice bearing BxPC3 pancreatic adenocarcinoma cells (integrin avb6–positive) or 293 (integrin avb6–negative) tumors. At 0.5, 1, and 2 h after injection, five-minute static scans were acquired. Coronal and transverse slices are presented. Tumor (T) and kidneys (K) are signed on images[24].
2) Targeting αvβ3 integrin
3) Targeting Hsp90
Heat shock protein 90 (Hsp90) plays an important role in the progress of malignant disease and elevated Hsp90 expression has been reported in pancreatic cancer. It resides exclusively in the cytosol in normal cells, but is activated and then removes to the cell surface in tumor cells [28, 29]. Furthermore, Hsp90 inhibitors selectively kill cancer cells compared to normal cells, it was reported that Hsp90 derived from tumour cells has a 100-fold higher binding affinity for the Hsp90 inhibitor 17-allylaminogeldanamycin (17-AAG) than does Hsp90 from normal cells [30]. Therefore, Hsp90 is an attractive target for cancer imaging [31, 32]. we found the most powerful Sansalvamide A derivative (IC501-20nM) , and radiolabeled 64Cu-Di-San A1 (Fig.5) for PET imaging of Hsp90 expression in a mouse model of pancreatic cancer. 64Cu-Di-San A1 was successfully prepared in a radiochemical yield >97% with a radiochemical purity >98%. Micro PET study shows good in vivo performance in terms of tumor uptake in nude mice bearing pancreatic cancer. The Hsp90-specifc tumor activity accumulation of 64Cu-Di-San A1 was further demonstrated by significant reduction of PL45 tumor uptake with pre-injected an Hsp90 inhibitor (17AAG). The ex vivo PET imaging and biodistribution results were consistent with the quantitative analysis of PET imaging, demonstrating good tumor-to-muscle ratio (5.35±0.46) at 4 h post-injection in PL45 tumor mouse xenografts. 64Cu-Di-San A1 allows PET imaging of Hsp90 expression in PL45 tumors, which may provide a non-invasive method to quantitatively characterize Hsp90 expression in pancreatic cancer [33].
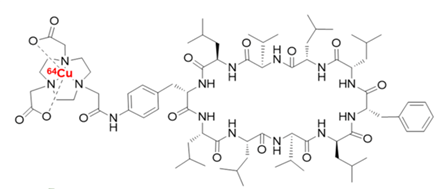
Fig. 5 The chemical structure of 64Cu-Di-San A1
Table 1 Radiosynthesis of peptides based PET Tracers for pancreatic cancer
|
Compounds |
Target |
Synthesis time |
Clinical available |
Specific activity (%ID/g) |
Different ratios |
HPLC needed |
Radiochemical yield (%) |
Ref |
|
[18F]FBA-A20FMDV2 [18F]FBA-PEG28-A20FMDV2 [18F]FBA-(PEG28)2-A20FMDV2 |
αvβ6 |
130 min |
No |
0.69 ± 0.19 1.85 ± 0.44 1.57 ± 0.25 |
tumor-to-background (1h:2.2:1 ; 3h :3.5:1) tumor-to-blood >47:1 |
Yes
|
3.6% _ _ |
|
|
18Ffluorobenzoate-R01
18F-fluorobenzoate-S02 |
45 min |
1.4 ± 0.6 0.5 ± 0.2 |
tumor-to-muscle 3.1±1.0 2.9 ±0.4 |
Yes |
23% ±13% |
[24] |
||
|
68Ga-NODAGA-RGD
|
Αvβ3 |
5min |
Clinical trial (Phase I) |
2-10 |
__
|
No |
> 96% |
[25] |
|
64Cu-RAFT-RGD |
60 min |
No |
6.01 ±0.75 |
Tumor to blood: 46.64 ± 9.93 Tumor to- muscle 9.3 ± 0.25 |
Yes |
>98% |
[27] |
|
|
64Cu-Di-San A1 |
Hsp90 |
2 h |
No |
2.97±0.58 |
tumor-to-muscle 5.35±0.46 |
Yes |
>97% |
[33] |
Targeting specific genes
G-protein-coupled cholecystokinin B receptor (CCKBR) was constitutively expressed on the surface of PDAC cells. Clawson et al.[34] described selection and characterization of high-affinity DNA aptamers (APs) to the CCKBR. Moreover, the uptake was increased in vivo of orthotopic PDAC tumors compared with native ligand gastrin. One AP, named AP1153 was chosen for further studies. They found that AP1153 was internalized by PDAC cells in a receptor-mediated manner. Bioconjugation of AP1153 to the surface of fluorescent NPs greatly facilitated delivery of NPs to PDAC tumors in vivo. The AP-targeted NP delivery system has potential for enhanced early detection of PDAC lesions [34].
The majority of patients with PDAC carry mutant KRAS2 oncogenes, and KRAS2 mRNA was activated and overexpressed in pancreatic cancer cells [35, 36]. Early detection of activated specific KRAS2 mRNAs in PDAC in vivo would be feasible by molecular imaging [37-39]. These probes are designed to bind to internalize and hybridize with KRAS oncogene mRNA that is overexpressed in pancreatic cancer.
Pancreatic ductal adenocarcinoma (PDAC) is the most representative type of pancreatic cancer. It begins in the cells lining the pancreatic duct. PDAC solid tumors are composed of heterogeneous populations of cells including cancer stem cells, differentiated cancer cells ( high-grade, moderate and worst differentiation), desmoplastic stroma and immune cells[40]. Although overall survival rates have improved for most cancers, pancreatic cancer is still currently most lethal malignancy[12, 41]. It has lowest survival rates among cancers, its deaths have not been decreasing over the past few years. Its 5-year survival rate is constant at a 6%, with more than 80% mortality within a year of diagnosis [1, 42, 43]. The lack of early diagnosis and ineffective treatment for advanced tumors are primarily the cause of the highly mortality. PDAC is on track to become the second most common cause of cancer-related deaths by 2030 in the United States[44].
Early pancreatic cancers often present atypical signs or symptoms. By the time they do cause symptoms, they have often already spread outside the pancreas. Jaundice is usually a typical symptom when the mass is located at head of pancreas. There are numerous risk factors cause pancreatic cancer. However, many people who get the disease may seldom have known risk factors.
Imaging is critical for the detection, characterization, management of pancreatic cancer cases [45, 46]. Due to the limitations of current anatomical imaging techniques, early detection of pancreatic cancer remains a field requires further improvement. Compared to conventional anatomical imaging technique modalities, PET is a new emerged and functional imaging tool that can offer the possibility of quantification of diseases associated biochemical processes[47]. It is commonly used for cancer diagnosis and staging, as well as offering prognostic information. Due to its low spatial resolution, pure PET imaging is subsequently combined with an X-ray CT scanner, which is able to overcome the drawback of PET. Thus, functional imaging obtained by PET, which depicts the spatial distribution of metabolic or biochemical activity in the body can be more precisely aligned with anatomic imaging obtained by CT scanning, and both functional and anatomical information are presented simultaneously in the same image. Other than traditional imaging modalities, injection of molecular imaging agents in the tested subject are required in order to acquire the PET imaging signals. Based on diverse principles, different agents may appear different images to the same lesion of the subject.
In this review, five general categories of PET imaging agents were examined, each tracer has its advantage and disadvantage, when designing novel research studies involving pancreatic cancer diagnosis, the current limitations of each category tracer should be considered. Taken together these tracers or probes represent promising methods for the establishment of novel imaging agents in the future. Meanwhile, the molecular markers mentioned in the review are also attractive targets for pancreatic cancer therapy when a precursor is labeled with long half-life radionuclide, such as 177Lu (t1/2:6.73d) and 89Zr (t1/2:78.4 h).
18F-FDG is the solely globally most commonly used imaging tracer in clinic, it is regarded as the “molecule of the century” in nuclear medicine. Nowadays, it is mainly used for oncology, which has great superiority in accurate staging, assessment of the therapeutic response and detection of recurrences. But as for diagnosis of tumor itself, 18F-FDG has its intrinsic limits. In order to overcome the overlaps between malignancy and benign lesions, dual-time-point-imaging (DPI)--at approximately 1 hour (early) and 2 hours (delayed) after injection are recommended [48, 49]. Delayed phase of 18F-FDG imaging may increase primary lesion detectability due to higher FDG uptake in primary tumors compared to the early phase of imaging [50, 51]. DPI may also help to differentiate between inflammatory and malignant lymph nodes [52]. However, it increased sensitivity for lesion detection (compromised specificity). There was study declared overall accuracy of DPI FDG PET/CT were better than that of single phase for less than 25 mm tumor, it might be useful for diagnosing small pancreatic tumors [49]. Besides, PET with enhanced CT will not only play an important role in differential diagnosis, but also give additional information concerning peripheral blood vessel. Whereas, in most PET/CT centers, only non-enhanced CT scans are undertaken.
Although numerous molecular imaging agents either directly measure metabolism of cells or bind to the overexpressed specific targets had been developed, only very few are translated into clinic for the diagnosis of pancreatic cancer. Most of these imaging modalities remain highly debatable and uncertain, which may be resolved through concerted cooperation from basic researchers in combination with radiologists and multicenter clinical trials [53]. Targeting of cell surface receptors overexpressed in cancer remains the most promising strategy for designing molecular imaging probes. A novel imaging probe with clinical translation potential is supposed to have the following unique characteristics: High binding affinity and specificity to target; High sensitivity, contrast ratio and stability in vivo; Low immunogenicity and toxicity; Production and economical feasibility[47]. Considering the liver is the most common metastatic organ for pancreatic cancer, hence to explore novel imaging agents with lower live uptake is also very important. In addition, improved instrumentation with high spatial resolution and lower expenditure is also should be considered. As for tumor models, orthotopic models probably reflect the physiological character of pancreatic cancer. Currently, most animal models in researches are subcutaneous.
In the future, molecular imaging will pave a pathway to both personalized medicine and precision medicine. Patients at risk for pancreatic cancer may be screened using highly optimal imaging agents and early detection is feasible to save millions of lives. However, great efforts are remain required to explore new imaging tracers that can overcome drawbacks of currently used agents and push more and more novel agents to serve to more patients in clinic.
This work was supported by the Lanzhou University Second Hospital Cuiying Youth Fund of China (CY-NQ-02).
Yabar CS, Winter JM. Pancreatic Cancer: A Review. Gastroenterology clinics of North America. 2016;45:429-45. PMid:27546841
View Article PubMed/NCBIDebebe SA, Goryawala M, Adjouadi M, McGoron AJ, Gulec SA. 18F-FLT Positron Emission Tomography/Computed Tomography Imaging in Pancreatic Cancer: Determination of Tumor Proliferative Activity and Comparison with Glycolytic Activity as Measured by 18F-FDG Positron Emission Tomography/Computed Tomography Imaging. Molecular imaging and radionuclide therapy. 2016;25:32-8. PMid:27299286 PMCid:PMC4807347
View Article PubMed/NCBIPiraka C, Scheiman JM. New diagnostic imaging modalities for pancreatic disease. Current opinion in gastroenterology. 2011;27:475-80. PMid:21743318
View Article PubMed/NCBIDimastromatteo J, Brentnall T, Kelly KA. Imaging in pancreatic disease. Nature reviews Gastroenterology & hepatology. 2016.
View ArticleFeldman MK, Gandhi NS. Imaging Evaluation of Pancreatic Cancer. The Surgical clinics of North America. 2016;96:1235-56. PMid:27865275
View Article PubMed/NCBIGrant TJ, Hua K, Singh A. Molecular Pathogenesis of Pancreatic Cancer. Progress in molecular biology and translational science. 2016;144:241-75. PMid:27865459
View Article PubMed/NCBIChen K, Conti PS. Target-specific delivery of peptide-based probes for PET imaging. Advanced drug delivery reviews. 2010;62:1005-22. PMid:20851156
View Article PubMed/NCBIRijkers AP, Valkema R, Duivenvoorden HJ, van Eijck CHJ. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: A meta-analysis. European Journal of Surgical Oncology.40:794-804.
View ArticleQuon A, Chang ST, Chin F, Kamaya A, Dick DW, Loo BW, Jr., et al. Initial evaluation of 18F-fluorothymidine (FLT) PET/CT scanning for primary pancreatic cancer. European journal of nuclear medicine and molecular imaging. 2008;35:527-31. PMid:17960376
View Article PubMed/NCBILamarca A, Asselin M-C, Manoharan P, McNamara MG, Trigonis I, Hubner R, et al. 18F-FLT PET imaging of cellular proliferation in pancreatic cancer. Critical Reviews in Oncology/Hematology. 2016;99:158-69. PMid:26778585
View Article PubMed/NCBIvan Waarde A, Cobben DC, Suurmeijer AJ, Maas B, Vaalburg W, de Vries EF, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2004;45:695-700.
England CG, Hernandez R, Eddine SB, Cai W. Molecular Imaging of Pancreatic Cancer with Antibodies. Molecular pharmaceutics. 2016;13:8-24. PMid:26620581
View Article PubMed/NCBIRashidian M, Keliher E, Dougan M, Juras PK, Cavallari M, Wojtkiewicz GR, et al. The use of 18F-2-fluorodeoxyglucose (FDG) to label antibody fragments for immuno-PET of pancreatic cancer. ACS central science. 2015;1:142-7. PMid:26955657
View Article PubMed/NCBIter Weele EJ, Terwisscha van Scheltinga AG, Kosterink JG, Pot L, Vedelaar SR, Lamberts LE, et al. Imaging the distribution of an antibody-drug conjugate constituent targeting mesothelin with (8)(9)Zr and IRDye 800CW in mice bearing human pancreatic tumor xenografts. Oncotarget. 2015;6:42081-90. PMid:26536664
View Article PubMed/NCBIWang H, Li D, Liu S, Liu R, Yuan H, Krasnoperov V, et al. Small-Animal PET Imaging of Pancreatic Cancer Xenografts Using a 64Cu-Labeled Monoclonal Antibody, MAb159. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56:908-13. PMid:25908833
View Article PubMed/NCBIZhang L, Li Z, Shi T, La X, Li H, Li Z. Design, purification and assessment of GRP78 binding peptide-linked Subunit A of Subtilase cytotoxic for targeting cancer cells. BMC biotechnology. 2016;16:65. PMid:27585649
View Article PubMed/NCBIEngland CG, Kamkaew A, Im HJ, Valdovinos HF, Sun H, Hernandez R, et al. ImmunoPET Imaging of Insulin-Like Growth Factor 1 Receptor in a Subcutaneous Mouse Model of Pancreatic Cancer. Molecular pharmaceutics. 2016;13:1958-66. PMid:27054683
View Article PubMed/NCBIHong H, Zhang Y, Nayak TR, Engle JW, Wong HC, Liu B, et al. Immuno-PET of tissue factor in pancreatic cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:1748-54. PMid:22988057
View Article PubMed/NCBIGirgis MD, Kenanova V, Olafsen T, McCabe KE, Wu AM, Tomlinson JS. Anti-CA19-9 Diabody as a PET Imaging Probe for Pancreas Cancer. Journal of Surgical Research. 2011;170:169-78. PMid:21601881
View Article PubMed/NCBIHausner SH, DiCara D, Marik J, Marshall JF, Sutcliffe JL. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin alphavbeta6 expression with positron emission tomography. Cancer research. 2007;67:7833-40. PMid:17699789
View Article PubMed/NCBIUeda M, Fukushima T, Ogawa K, Kimura H, Ono M, Yamaguchi T, et al. Synthesis and evaluation of a radioiodinated peptide probe targeting alphavbeta6 integrin for the detection of pancreatic ductal adenocarcinoma. Biochemical and biophysical research communications. 2014;445:661-6. PMid:24583127
View Article PubMed/NCBIGao D, Gao L, Zhang C, Liu H, Jia B, Zhu Z, et al. A near-infrared phthalocyanine dye-labeled agent for integrin alphavbeta6-targeted theranostics of pancreatic cancer. Biomaterials. 2015;53:229-38. PMid:25890722
View Article PubMed/NCBIHausner SH, Abbey CK, Bold RJ, Gagnon MK, Marik J, Marshall JF, et al. Targeted in vivo imaging of integrin alphavbeta6 with an improved radiotracer and its relevance in a pancreatic tumor model. Cancer research. 2009;69:5843-50. PMid:19549907
View Article PubMed/NCBIHackel BJ, Kimura RH, Miao Z, Liu H, Sathirachinda A, Cheng Z, et al. 18F-fluorobenzoate-labeled cystine knot peptides for PET imaging of integrin alphavbeta6. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:1101-5. PMid:23670900
View Article PubMed/NCBITrajkovic-Arsic M, Mohajerani P, Sarantopoulos A, Kalideris E, Steiger K, Esposito I, et al. Multimodal molecular imaging of integrin alphavbeta3 for in vivo detection of pancreatic cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:446-51. PMid:24549287
View Article PubMed/NCBIHaubner R, Finkenstedt A, Stegmayr A, Rangger C, Decristoforo C, Zoller H, et al. [(68)Ga]NODAGA-RGD - Metabolic stability, biodistribution, and dosimetry data from patients with hepatocellular carcinoma and liver cirrhosis. European journal of nuclear medicine and molecular imaging. 2016;43:2005-13. PMid:27164900
View Article PubMed/NCBIAung W, Jin ZH, Furukawa T, Claron M, Boturyn D, Sogawa C, et al. Micro-positron emission tomography/contrast-enhanced computed tomography imaging of orthotopic pancreatic tumor-bearing mice using the alphavbeta(3) integrin tracer (6)(4)Cu-labeled cyclam-RAFT-c(-RGDfK-)(4). Molecular imaging. 2013;12:376-87. PMid:23981783
View Article PubMed/NCBISnigireva AV, Vrublevskaya VV, Afanasyev VN, Morenkov OS. Cell surface heparan sulfate proteoglycans are involved in the binding of Hsp90alpha and Hsp90beta to the cell plasma membrane. Cell adhesion & migration. 2015;9:460-8. PMid:26651243
View Article PubMed/NCBIOgata M, Naito Z, Tanaka S, Moriyama Y, Asano G. Overexpression and localization of heat shock proteins mRNA in pancreatic carcinoma. Journal of Nippon Medical School = Nippon Ika Daigaku zasshi. 2000;67:177-85. PMid:10851351
View Article PubMed/NCBIKamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407-10. PMid:14508491
View Article PubMed/NCBIKang J, Young Lee J, Tas I, More KN, Kim H, Park JH, et al. Radiosynthesis, biological evaluation and preliminary microPET study of (18)F-labeled 5-resorcinolic triazolone derivative based on ganetespib targeting HSP90. Bioorganic & medicinal chemistry letters. 2018;28:3658-64. PMid:30528977
View Article PubMed/NCBITaldone T, Zatorska D, Ochiana SO, Smith-Jones P, Koziorowski J, Dunphy MP, et al. Radiosynthesis of the iodine-124 labeled Hsp90 inhibitor PU-H71. Journal of labelled compounds & radiopharmaceuticals. 2016;59:129-32. PMid:26806023
View Article PubMed/NCBIWang X, Zhang J, Wu H, Li Y, Conti PS, Chen K. PET imaging of Hsp90 expression in pancreatic cancer using a new (64)Cu-labeled dimeric Sansalvamide A decapeptide. Amino acids. 2018;50:897-907. PMid:29691700
View Article PubMed/NCBIClawson GA, Abraham T, Pan W, Tang X, Linton SS, McGovern CO, et al. A Cholecystokinin B Receptor-Specific DNA Aptamer for Targeting Pancreatic Ductal Adenocarcinoma. Nucleic acid therapeutics. 2016.
View ArticleMorrison M. Pancreatic cancer and diabetes. Advances in experimental medicine and biology. 2012;771:229-39.
View ArticleGrigor'eva IN, Efimova OV, Suvorova TS, Tov NL. [Genetic aspects of pancreatic cancer]. Eksperimental'naia i klinicheskaia gastroenterologiia = Experimental & clinical gastroenterology. 2014:70-6.
Amirkhanov NV, Zhang K, Aruva MR, Thakur ML, Wickstrom E. Imaging human pancreatic cancer xenografts by targeting mutant KRAS2 mRNA with [(111)In]DOTA(n)-poly(diamidopropanoyl)(m)-KRAS2 PNA-D(Cys-Ser-Lys-Cys) nanoparticles. Bioconjugate chemistry. 2010;21:731-40. PMid:20232877
View Article PubMed/NCBICheng KT, Chakrabart A, Aruva MR, Thakur ML, Wickstrom E. 64Cu-N,N'-Bis(S-benzoyl-thioglycoloyl)diaminopropanoate-KRAS-PNA-d(Cys-Ser-Lys-Cy s). Molecular Imaging and Contrast Agent Database (MICAD). Bethesda (MD): National Center for Biotechnology Information (US); 2004.
Chakrabarti A, Aruva MR, Sajankila SP, Thakur ML, Wickstrom E. Synthesis of novel peptide nucleic acid-peptide chimera for non-invasive imaging of cancer. Nucleosides, nucleotides & nucleic acids. 2005;24:409-14.
View ArticleCalle AS, Nair N, Oo AK, Prieto-Vila M, Koga M, Khayrani AC, et al. A new PDAC mouse model originated from iPSCs-converted pancreatic cancer stem cells (CSCcm). American journal of cancer research. 2016;6:2799-815.
Mustafa S, Pan L, Marzoq A, Fawaz M, Sander L, Ruckert F, et al. Comparison of the tumor cell secretome and patient sera for an accurate serum-based diagnosis of pancreatic ductal adenocarcinoma. Oncotarget. 2017.
View ArticleSharib J, Kirkwood K. Early and accurate diagnosis of pancreatic cancer? Oncotarget. 2016.
View ArticleChampanhac C, Teng IT, Cansiz S, Zhang L, Wu X, Zhoa Z, et al. Development of a panel of DNA Aptamers with High Affinity for Pancreatic Ductal Adenocarcinoma. Scientific reports. 2015;5:16788. PMid:26603187 PMCid:PMC4658478
View Article PubMed/NCBIYing H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes & development. 2016;30:355-85. PMid:26883357
View Article PubMed/NCBIDenbo JW, Fleming JB. Definition and Management of Borderline Resectable Pancreatic Cancer. The Surgical clinics of North America. 2016;96:1337-50. PMid:27865281
View Article PubMed/NCBIFric P, Skrha J, Sedo A, Zima T, Busek P, Kmochova K, et al. Early detection of pancreatic cancer: impact of high-resolution imaging methods and biomarkers. European journal of gastroenterology & hepatology. 2016;28:e33-e43.
View ArticleChen K, Chen X. Design and development of molecular imaging probes. Current topics in medicinal chemistry. 2010;10:1227-36. PMid:20388106
View Article PubMed/NCBIMena E, Sheikhbahaei S, Taghipour M, Jha AK, Vicente E, Xiao J, et al. 18F-FDG PET/CT Metabolic Tumor Volume and Intratumoral Heterogeneity in Pancreatic Adenocarcinomas: Impact of Dual-Time Point and Segmentation Methods. Clinical nuclear medicine. 2017;42:e16-e21.
View ArticleKawada N, Uehara H, Hosoki T, Takami M, Shiroeda H, Arisawa T, et al. Usefulness of dual-phase 18F-FDG PET/CT for diagnosing small pancreatic tumors. Pancreas. 2015;44:655-9. PMid:25815646
View Article PubMed/NCBISurucu E, Polack BD, Demir Y, Durmusoglu M, Ekmekci S, Sarioglu S, et al. Dual-phase F-18 FDG PET-CT in staging and lymphoscintigraphy for detection of sentinel lymph nodes in oral cavity cancers. Clinical imaging. 2015;39:781-6. PMid:25721710
View Article PubMed/NCBIXu XX, Cheng J, Xu WG, Dai D, Song XY, Ma WC, et al. [Value of dual-time-point (18)F-fluorodeoxyglucose integrated positron emission and computed tomography in differentiation of malignant from benign gastrointestinal diseases]. Zhonghua zhong liu za zhi [Chinese journal of oncology]. 2012;34:364-8.
Mayoral M, Paredes P, Domenech B, Fuste P, Vidal-Sicart S, Tapias A, et al. 18F-FDG PET/CT and sentinel lymph node biopsy in the staging of patients with cervical and endometrial cancer. Role of dual-time-point imaging. Revista espanola de medicina nuclear e imagen molecular. 2017;36:20-6. PMid:27667001
View Article PubMed/NCBIPhillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Science translational medicine. 2014;6:260ra149.
View Article