Vânia Margaret Flosi Paschoalin, D.Sc.
Avenida Athos da Silveira Ramos 149, Cidade Universitária, 21 941-909, RJ, Brazil.
Phone: 55 21 3938 7362. Fax: 55 21 3938 7266.
E-mail: paschv@iq.ufrj.br
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 6
Page No: 484-496
Vânia Margaret Flosi Paschoalin, D.Sc.
Avenida Athos da Silveira Ramos 149, Cidade Universitária, 21 941-909, RJ, Brazil.
Phone: 55 21 3938 7362. Fax: 55 21 3938 7266.
E-mail: paschv@iq.ufrj.br
Diego dos Santos Baião1, Fabrício de Oliveira Silva1, Jenifer d’El-Rei2, Mario Fritsch Neves2, Daniel Perrone1, Eduardo Mere Del Aguila1 and Vânia Margaret Flosi Paschoalin1,*
1Instituto de Química, Universidade Federal do Rio de Janeiro, Avenida Athos da Silveira Ramos 149, 21941-909, Rio de Janeiro – RJ, Brazil.
2Departamento de Clínica Médica, Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro, Boulevard 28 de Setembro 77, 20551-030, Rio de Janeiro - RJ, Brazil.
Ana Paula Bettencourt(abete@quimica.uminho.pt)
M A Darenskaya MA(marina_darenskaya@inbox.ru)
C T Hern%c3%a1ndez-Delgado CT(tzasna@unam.mx)
Chong Tian C(tianchong0826@hust.edu.cn)
Vânia Margaret Flosi Paschoalin, A new functional beetroot formulation enhances adherence to nitrate supplementation and health outcomes in clinical practice(2018)SDRP Journal of Food Science & Technology 3(6)
Background: Beetroot has been used as a source of dietary nitrate supplementation, a precursor for the endogenous synthesis of nitric oxide, which improves endothelial function and displays protective effects against cardiovascular diseases.
Methods: The aim of this study was to formulate an attractive and well-tolerated beetroot-derivative containing high levels of nitrate and antioxidant compounds, able to reduce blood pressure in hypertensive patients following chronic ingestion. A beetroot-cereal bar was prepared from a mixture of beetroot juice, beetroot chips and cereals. Sixty grams of the beetroot-cereal bar were administered to five hypertensive patients recruited from a cardiovascular medical service for 3-week supplementation. At the end of this period, the patients were examined at the routine hospital clinical service at 8:20 AM after a rest of 20 min.
Results: The nitrate contents of the beetroot-cereal bar were 15.3±0.05 mmol∙100 g-1, which also contained 8.64±1.85 mg∙100 g-1 of saponins, 9.19±0.71 mg∙g-1 of organic acids and 147.73±3.3 mg∙100 g-1 of phenolic compounds. Beetroot-cereal bars displayed a shelf life of at least 30 days with no preservative addition. A rheological evaluation of the beetroot-cereal bars indicated the predominance of a dark red color and crispy texture. The beetroot-cereal bar displayed high overall acceptability and purchase intent. The chronic consumption of beetroot-cereal bar by hypertensive patients led to ≈-14.0 mm Hg decrease in systolic and ≈-6.5 mm Hg decrease in diastolic blood pressures.
Conclusion: Beetroot-cereal bars may be a new attractive strategy to offer high levels of bioaccessible dietary nitrate and antioxidant compounds that can aid in the improvement of cardiovascular function.
Keywords: beetroot-cereal bar, antioxidant activity, rheological characteristics, blood pressure, chronic consumption.
Foods of vegetable origin have been intensively investigated due to certain compounds that give them their taste, aroma and nutritional value. In addition, nutrients that provide physiological benefits for the promotion and maintenance of human health, termed functional nutrients [1], are also present in the vegetable matrix. However, only a small number of bioactive nutrients from vegetable sources have been tested in clinical trials and displayed evident health benefits, as well as surpassed the rigorous standard for “significant scientific agreement” required by the FDA for the authorization of health claims [2]. Beetroot (Beta vulgaris L. species), a member of the Quenopodiaceae family, generally known as red or garden beetroot, exhibits high nutritional value and is considered a high dietary source of fibers, antioxidants, vitamins and minerals. Approximately 85% of dietary nitrate (NO3-) comes from vegetables, and beetroot is considered one of the most significant sources of dietary NO3- [3,4].
Dietary NO3- is a precursor for the endogenous synthesis of nitric oxide (NO), a major vasodilator synthesized by the endothelium. In this context, beetroot consumption has been associated with several health benefits, such as the improvement of endothelial function, decreased blood pressure in normal and hypertensive individuals [5], physiological reduction of cerebral vascular resistance, increased tissue oxygenation and performance improvement, as well as increased tolerance to fatigue during physical exercise [6].
To promote the stimulation of NO production and obtain protective effects against cardiovascular disease (CVD), NO3- contents, ranging from 7.0 mmol (310 mg) to 28.6 mmol (1773 mg), found in different beetroot formulations should be administered (3,4,7]. However, to reach the effective NO3- concentration, some of these formulations must be offered in large volumes, making it difficult for individuals to adhere to the proposed intervention. Moreover, it is a challenge to provide a product which, besides being rich in NO3-, must also be attractive in appearance, taste and smell, easily administered, and adequate in size portion and format.
Cereal bars are included in the snack or snack-food category and have gained significant popularity in the market, as they can be formulated from the compaction of dehydrated fruits and cereals, such as oats, wheat, soybeans, corn and rice [8] and are suitableas a source of high nutrient concentrations in small amounts of product.
In this context, the present study aimed to design a beetroot formulation to be used as a dietary NO3- source supplementation and assessits effects on the blood pressure of subjects presenting risk factors for the development of CVD following chronic administration. Beetroot-cereal bar physico-chemical and biochemical characteristics were assessed. Product shelf life, sensorial evaluation, and rheological properties were also determined, in order to evaluate consumer acceptance and purchase intent.
Standards and reagents
Standards solutions and reagents were purchased from Sigma-Aldrich Chemical Co. (MO, USA). All solvents were of high-performance liquid chromatography (HPLC) grade and purchased from Tedia Company Inc. (OH, USA). Brilliant green bile broth (Rappaport), Escherichia coli LB medium (BD™), violet red bile agar, mannitol egg yolk polymyxin agar, and potato dextrose agar were purchased from Hexis Científica (SP, BRA). 3M Salmonella enrichment base and supplement and PetrifilmTM Salmonella express (SALX) plates were purchased from 3M Co. (MN, USA). HPLC grade Milli-Q water (Merck Millipore, MA, USA) was used throughout the experiments.
Beetroot-cereal bar formulation
All ingredients used in the beetroot formulation were purchased from the local trade in the Rio de Janeiro municipality, Southeastern Brazil. Beetroots (Beta vulgaris L.) with no signs of deterioration were selected, such as cracks, stains, bruises, or wet areas. Approximately 4 kg of beetroots were carefully packed in black sealed plastic bags and stored at 5°C for 2 days, in order to avoid damage and microbial spoilage. The beetroot juice was prepared as described previously [9]. Beetroot chips were produced according to Vasconcellos et al. [7]. The beetroot-cereal bar was prepared by mixing the ingredients from the ligand and dry phases. The ligand phase containing beetroot juice (with no water addition), brown sugar (40 g), corn syrup (50 g) and citric acid (1 g) was transferred to a stainless steel pot, dispersion was performed in a water bath at 90°C for 15 min and the mixture was then cooled at room temperature. After 10 min, beetroot powder was obtained from crushed chips (300 g) and other ingredients, such as rolled oats (150 g), whole oats (150 g), rice flakes (60 g) and honey (40 g), were homogenously added.
The cereal bar matrix was distributed in a stainless-steel container (30 cm x 20 cm) and pressed until reaching a thickness of 1.5 cm. The beetroot-cereal matrix was then baked at 200°C for 15 min and cut into 20 pieces, obtaining beetroot-cereal bars measuring 10 cm x 3 cm x 1.5 cm.
Beetroot-cereal bar analyses
All beetroot-cereal bar biochemical and microbiological analyses were performed in triplicate, and the results were expressed as 100 g of dry weight basis (dwb).
Rheological analyses
Texture
The texture of the beetroot-cereal bar was evaluated according to Mattos et al. [10], using a Texture Analyser Stable Micro Systems model (Waverley, UK).
Color
The color intensity of the beetroot-cereal bar was evaluated according to Chandran et al. [11], using an Ultra Scan Vis Colorimeter (Hunter Lab, VA, USA).
Proximate composition
Moisture, ash, protein, lipid and total dietary fiber contents were determined in the beetroot-cereal bar according to the Association of Official Analytical Chemists [12]. Total carbohydrates were estimated by deducting the sum of moisture, ash, protein, lipid and total dietary fibers from 100%. Calorific values (kcal) were calculated from the approximate chemical composition data using the general factor system.
Sugar analysis
Fructose, glucose, sucrose and maltose contents in the beetroot-cereal bar were evaluated as described previously [13]. Samples were injected into a high-performance liquid chromatography (HPLC) system LC-20AD (Shimadzu®, Kyoto, JPN). Chromatographic separation of sugars was achieved using an NH2 column (5 mm, 250 × 4.6 mm, I.D., Zorbax®, CA, USA) and isocratic elution (82% acetonitrile in distilled and deionized H2O) at a 1.0 mL∙min-1 flow rate. Sugars were detected using a refractive index detector RID-10A (Waters, MA, USA) coupled to a signal integrator CBM-20A (Shimadzu®) (supplementary file 1).
NO3- and NO2- analyses
The NO2- and NO3- analyses were performed as described previously [9]. The HPLC device was equipped with a 5 μm reversed-phase C8 column (150 x 4.6 mm, I.D., Ascentis®, CA, USA), a 5 μm reversed-phase C18 guard column (50 x 4.6 mm, I.D., Ascentis®, CA, USA) and a fluorescence detector model RF-10AXL (Shimadzu®). Fluorescence was monitored at excitation and emission wavelengths of 375 nm at 415 nm. Sodium phosphate buffer (pH 7.5) at 15 mmol∙L-1 and methanol (50:50, v/v) were used as the mobile phase for gradient elution at a 1.3 mL∙min-1 flow rate (supplementary file 2).
Saponin analyses
Total saponin content was determined by the vanillin-sulfuric acid assay [14]. Absorbances at 535 nm were recorded using a V–530 UV/VIS spectrophotometer (Jasco®, Tokyo, JPN).
Organic acid determinations
Organic acids were determined as described by Leite et al. [15]. Filtered beetroot samples were injected into the HPLC system (Shimadzu Corp.) equipped with an HPX-87H Aminex fermentation monitoring column (150 × 7.8 mm, I.D., Bio-Rad Laboratories Inc.) and protected by a cation H+Micro-Guard cartridge (30 × 4.6 mm, I.D., Bio-Rad Laboratories Inc.), maintained at 65°C. Organic acids were detected using an SPD-M20A diode array detector model (Shimadzu Corp.) at 210 nm (supplementary file 3).
Determination of phenolic compounds (PC)
PC extraction from the beetroot-cereal bar was performed according to a methodology adapted from Inada et al. [13], using soluble and conjugate phenolic extraction methods. All extracts were filtered through 0.45 µm cellulose ester-membranes (Merck Millipore Co.) prior to the HPLC analyses.
The HPLC device was equipped with a 5 μm reversed-phase C18 column (250 x 4.6 mm, I.D., Ascentis®) guarded by a 5 μm C18 guard column (10 x 3.0 mm, I.D., Ascentis®) and comprising an SPD-M30A photodiode array (PDA) detector (Shimadzu Corp.) with readings ranging from 190 nm to 370 nm. The column temperature was set at 40°C and the injection volume was 20 µL for all samples. The mobile phase (1.0 mL∙min-1) was composed of 0.3% formic acid (in H2O-DD), methanol (100%) and acetonitrile (100%) at a gradient elution (supplementary file 4).
Total antioxidant potential determination (TAP)
The beetroot-cereal bar was analyzed as previously described by da Silva et al. [16]. Samples were transferred to amber vials and incubated at 37°C for 10 min with a solution containing 1 mM Fe2+, 10 mM H2O2 and 1 mM terephthalic acid (TPA) in 50 mM phosphate buffer (pH 7.4). The hydroxyterephthalic acids (HTPA) were detected by HPLC. TAP measurements were obtained by the difference between the chromatogram surface area generated in the Fenton reaction with and without the sample (supplementary file 5).
Antioxidant activity determination by different assays
Ferric reducing ability of plasma (FRAP)
FRAP assays were performed by a modification of the method described by Benzie and Strain [17]. Absorbances at 593 nm were determined using a V–530 UV/VIS spectrophotometer (Jasco®).
Trolox equivalent antioxidant capacity (TEAC)
TEAC assays were performed using a modification of the method described by Re et al. [18]. The beetroot sample was mixed with the ABTS•+ reagent and absorbances at 720 nm were determined using a V–530 UV/VIS spectrophotometer (Jasco®).
Oxygen radical antioxidant capacity (ORAC)
The ORAC assay was performed according to Zuleta et al. [19], with modifications. Sample absorbances were measured using a Wallac 1420 VICTOR multilabel counter (Perkin–Elmer Inc, MA, USA) with fluorescence filters at an excitation wavelength of 485 nm and emission wavelength of 535 nm.
Radical scavenging capacity 2.2-diphenyl-1-picrylhydrazyl (DPPH)
The DPPH assay was performed according to von Gadow et al. [20], with modifications. The absorbance was determined at 517 nm using a DU-530 spectrophotometer (Beckman Coulter Inc, IL, USA). The percentage of DPPH radical inhibition was calculated according to the following equation:
DPPH (% inhibition) = (1- (Abssample- Abssample control))/ Abscontrol test x 100
Lipid peroxidation inhibition
Lipid peroxidation analyses were carried out as described by Samaranayaka et al. [21]. Butyrate hydroxyanisole (BHA), butylated hydroxytoluene (BHT), α-tocoferol and beetroot samples were prepared in an emulsion system containing linoleic acid (1.3% in ethanol)/ethanol (75%)/H2O-DD). Flasks containing samples and standards were sealed and incubated at 40°C in a shaker for 7 days, without light interference. Absorbances were determined at 550 nm using a DU-530 spectrophotometer (Beckman Coulter Inc.).
Microbiological analyses
The beetroot-cereal bar was evaluated at 0, 15 and 30 days of storage at 15°C. Enumeration of yeast and mold in the saline solutions was performed according to the American Public Health Association [22]. The results were expressed as the number of colony-forming units per gram (CFU∙g-1). Salmonella spp. and Escherichia coli (E. coli) enumerations were assessed by the 3M PetrifilmTM Salmonella Express System and 3M PetrifilmTM E. coli Count Plate (3M Health Care), respectively. Total and fecal coliforms were assessed by standard methodology of the most likely number per gram (MPN∙g-1). Bacillus cereus were enumerated in selective MYP media (Plast Labor®, RJ, BRA), according to the American Public Health Association [22].
Sensorial evaluation and purchase intention
Sensorial evaluation procedures were performed in accordance with the ethical standards of the declaration of Helsinki and approved by the Institutional Ethics Committee of the Hospital Universitário Clementino Fraga Filho, Rio de Janeiro (No. 15510313.5.0000.5257). Beetroot-cereal bars were evaluated by one hundred untrained male and female panelists, using a 9-point hedonic scale. The evaluated sensory attributes were color, flavor, aroma, texture and overall acceptability. The purchase intention of the beetroot-cereal bars was assessed at the same time, using a 5-point hedonic scale [23].
Pilot study of the assessment of beetroot-cereal bar chronic intake effects
Five hypertensive women with a systolic blood pressure (SBP) > 140 mm Hg and diastolic blood pressure (DBP) > 95 mm Hg were recruited from the Hospital Universitário Pedro Ernesto (HUPE) at the Universidade do Estado do Rio de Janeiro (UERJ). Study participants were evaluated by anthropometry, blood pressure measurements and blood biochemical analyses. The exclusion criteria for participation in the study included any known pulmonary, and/or metabolic diseases (asthma, diabetes, smoking), elderly subjects (> 60 years-old), urinary infection, pregnancy, lactation, menstrual period and (or) the use of either nutritional and/or pharmacological ergogenics. Urinary infection was assessed by the presence of NO3- in urine using UriquestÒ Plus test strips (Labtest Diagnóstica S.A., Minas Gerais, Brazil). If volunteers were taking any medication, it would be withdrawn the afternoon before the clinical visit, to avoid interferences.
All hypertensive patients were fully informed of the nature and purpose of the study and gave their written consent to participate. All experimental procedures were performed at the HUPE under medical supervision and in accordance with the ethical standards of the Declaration of Helsinki. The study was approved by the local Ethics Committee, under No. CAAE 57774816.5.0000.5259.
The volunteers reported to the HUPE on two occasions. Both visits were held at 8 AM, according to the clinical service routine. The volunteers were advised to restrict the ingestion of foods rich in NO2- and NO3- for 42 h before visits and received a list describing foods and food groups to be avoided or preferred based on dietary NO2- and NO3- [16]. Subjects were also instructed to restrict the ingestion of carbonated water, the use of mouthwash, avoid alcohol and caffeine intake for 24 h and the practice of strenuous exercise and fasting at least 12 h before and during the evaluation.
All subjects received 3-week supplies of 60 g of beetroot-cereal bar enriched in dietary NO3- to consume in the afternoon, as a single snack, replacing other commonly consumed foods. The SBP and DBP, and heart rate (HR) were recorded before and after the 3-weeks of 60 g beetroot-cereal bar supplementation, by taking three supine blood pressure measurements of the brachial artery, at 1-min intervals, using an automated HEM-705CP Omron sphygmomanometer (Healthcare Inc., IL, USA) applied to the right arm after a 20 min rest (8:20 AM). All clinical tests were performed in a quiet and temperature-controlled environment.
Statistical analyses
A one-way (ANOVA) analysis of variance with repeated measures was performed to identify beetroot-cereal bar differences with respect to chemical composition, TAP, antioxidant activity, microbiological analyses, sensorial evaluations and rheological properties.
A two-way analysis of variance (ANOVA) 2 x 2 pathways with repeated measures for two factors (intervention x time: pre and post intervention) was performed to identify differences in SBP, DBP and HR parameters. When a significant F was found, an additional post hoc analysis was performed applying a Bonferroni correction. Results were considered significant when P<0.05. Data were expressed as the means ± standard deviation (SD). Statistical procedures were carried out using the Graphpad Prism software version 5 for Windows® (GraphPad Software, CA, USA).
Proximate composition and sugar content
The proximate composition of the beetroot-cereal bar comprised 16.20±0.39 g∙100 g-1 protein, 0.97±1.00 g∙100 g-1 lipids, 62.97±0.97 g∙100 g-1 carbohydrates and 325.58±2.5 kcal∙100 g-1 energy. The beetroot-cereal bar also contained 4.07±0.14 g∙100 g-1 total dietary fiber and low moisture (12.9±0.50%), as expected. Total sugar content of the beetroot-cereal bar was 37.72±0.70 g∙100 g-1 (Table 1).
The manufacturing of the beetroot-cereal bar, designed to supply NO3-, included binding phase ingredients, which increased carbohydrate and energy content but maintained low lipid concentrations. Lipids were the lowest contributor to beetroot-cereal bar energy (< 1 g∙100 g-1), thus classifying the bars as a low fat food, according to the current legislation for solid food in Brazil [24]. Furthermore, maintaining low lipid concentrations did not compromise flavor, texture or other organoleptic characteristics.
The addition of ingredients such as rolled oats, whole oat bran and rice flakes contributed to the increased protein and dietary fiber content of the beetroot-cereal bar. Thus, the beetroot-cereal bar can be considered a fiber source, in accordance with the current Brazilian legislation [24], containing dietary fiber amounts over 3 g∙100 g-1 of product. It is known that a high dietary fiber intake has been associated with decreased risks for coronary and CVD. Dietary fiber also decreases blood cholesterol levels, by increasing the capacity for bile acid or short chain fatty acid production and inhibiting the growth of pathogenic bacteria, by stimulating the development of beneficial bacterial flora, which increases immune system responses and prevents gastrointestinal infections [25].
High moisture in cereal bars can promote undesirable product modifications, such as non-enzymatic browning and crispness decreases, which are both important sensory attributes for these products [8]. The moisture content of the developed beetroot-cereal bar is within the established limits for cereal bars, which cannot exceed 15% [24].
| Table 1: Proximate composition, sugars, NO3-, NO2- and total saponin contents of the beetroot-cereal bar | |
|
|
Beetroot-cereal bar (100 g) |
|
Ash (%) |
15.3±0.06 |
|
Moisture (%) |
12.9±0.50 |
|
Energy (kilocalorie) |
325.58±2.5 |
|
Carbohydrate (g) |
62.97±0.97 |
|
Protein (g) |
16.20±0.39 |
|
Lipids (g) |
0.97±1.00 |
|
Total dietary fibers (g) |
4.07±0.14 |
|
Total sugars (g) |
37.72±0.7 |
|
Fructose (g) |
2.79±0.15 |
|
Glucose (g) |
4.71±0.16 |
|
Sucrose (g) |
26.59±0.80 |
|
Maltose (g) |
3.63±0.19 |
|
NO3- (mmol) |
1.3±0.05 |
|
NO2- (mmol) |
0.20±0.01 |
|
Saponins (mg) |
8.648±1.85 |
Values are expressed as means ± SD.
NO3- and NO2- contents
Beetroot-cereal bar NO3- content was of 15.3±0.05 mmol∙100 g-1 (Table 1). NO2- content, on the other hand, was very low, at 0.20±0.01 mmol∙100 g-1, with no physiological significance.
Clinical studies have reported that beetroot supplementation with dietary NO3- in concentrations ranging from 1.6 to 22 mmol seems to be sufficient to result in vascular benefits [6]. Systematic reviews and meta-analyses have evaluated vascular responses to NO3-, including decreased blood pressure, platelet aggregation inhibition, vascular endothelium preservation or endothelial dysfunction improvement during physical activity [26]. Evaluation of beetroot juice supplementation effects on blood pressure indicates decreases in systolic [-3.55 mm Hg; 95% CI: -4.55, -2.54 mm Hg] and diastolic [-1.32 mm Hg; 95% CI: -1.97, -0.68 mm Hg] blood pressure. Furthermore, decreases in systolic blood pressure were higher following longer supplementation periods compared to shorter periods (≥ 14 days compared to < 14 days of treatment) [-5.11 compared to -2.67 mm Hg] and observed for the highest administered volume compared to the lowest (500 mL compared to 70 mL and 140 mL/day) [-4.78 compared to -2.37 mm Hg] doses of beetroot juice supplementation [27].
These beneficial vascular function effects can be explained by the dietary NO3- found in beetroot that forms endogenous NO [3]. In the last few years, different beetroot formulations have been considered as adequate sources of NO3- to stimulate NO production. Juice, gel, raw or cooked beetroot are all able to supplement dietary NO3- [7]. However, some beetroot formulations must be offered in large serving portions to reach effective NO3- concentrations. Consumption of high beetroot volumes can lead to gastric discomfort, nausea and vomiting, and it is also difficult to convince individuals to adhere to certain proposed interventions, mainly long-term ones. The challenge is to provide a product that, in addition to being rich in NO3-, is attractive, easy to administer and microbiologically safe, containing bioactive compounds that aggregate nutritional value, in order to obtain a super supplement to improve beneficial NO3- health effects. Herein, a beetroot-based product formulated as a cereal bar was designed to provide a small and easily served portion as a healthy supplement for individuals in active CVD or at risk for CVD with effective doses of dietary NO3- in 100 g of food, higher than those in other beetroot-products, such as beetroot juice, chips and gel [7,16].
Saponin contents
Saponin content in the beetroot-cereal bar was 8.648±1.85 mg∙100 g-1 (Table 1). Saponins are considered antiviral, antidiabetic, cytotoxic and allelopathic agents. Several beneficial bioactivities are attributed to triterpene compounds, with the most important saponin effect observed on cholesterol absorption and metabolism, reducing cholesterol levels in the serum and liver of several animals, including humans [28].
Organic acid identification and quantification
The beetroot-cereal bar contained 9.19±0.71 mg∙g-1 of total organic acid content (Table 2). Six distinct organic acids were identified and quantified in the beetroot-cereal bar, namely, citric, ascorbic, malic, fumaric, succinic and oxalic acids. Malic and citric acid were the most abundant organic acids detected in the product.
Organic acids display effective bactericidal potential due to their ability to dissociate. Among the organic acids observed in the present study, ascorbic (pKa 4.76), malic (pKa 5.10), succinic (pKa 5.63) and citric (pKa 6.40) acids are capable of dissociating in the acidic gastrointestinal environment, resulting in hyper-acidification, with positive charges outside the microorganism, disrupting the proton-gradient across the plasma membrane and causing cell death [29]. These acids are also found in their non-dissociated forms (presenting lipophilic characteristics) and can easily diffuse through the bacterium membrane into the cell cytoplasm. Once inside the cell, where the pH is close to neutral, these organic acids are dissociated and release protons that acidify the cytoplasm, resulting in the dissolution of the proton-motor force by suppressing the enzymatic system, nutrient transport, amino acid and energy metabolism and DNA synthesis. In addition, the cationic portion of the organic acids released into the cell reduces the pH of the medium, forcing bacterial cells to use their energy to release protons, leading to exhaustion [29].
|
Table 2. Organic acids and phenolic contents of the beetroot-cereal bar |
|
||
|
Organic acid (mg∙g-1) |
Beetroot-cereal bar |
|
|
|
Citric acid |
2.31±0.14 |
|
|
|
Ascorbic acid |
1.55±0.21 |
|
|
|
Malic acid |
3.00±.0.1 |
|
|
|
Fumaric acid |
0.81±0.1 |
|
|
|
Succinic acid |
0.51±0.01 |
|
|
|
Oxalic acid |
0.50±0.15 |
|
|
|
Total |
9.19±0.71 |
|
|
|
Phenolic compounds (mg∙100 g-1) |
|
||
|
Vanillic acid |
13.14±0.108 |
|
|
|
p-Coumaric acid |
39.68±1.214 |
|
|
|
Rosmarinic acid |
4.25±0.039 |
|
|
|
3,4-Dihydroxybenzoic acid |
9.97±0.120 |
||
|
Gallic acid |
60.50±1.761 |
||
|
Syringic acid |
4.48±0.004 |
||
|
Caffeic acid |
5.94±0.033 |
||
|
Ferulic acid |
3.23±0.008 |
||
|
Chlorogenic acid |
5.69±0.008 |
||
|
Total |
147.73±3.3 |
||
Values are expressed as means ± SD.
Total phenolic compounds
The beetroot-cereal bar presented 147.73±3.3 mg∙100 g-1 of total phenolic compound content and a high diversity of these compounds (Table 2). Nine phenolic compounds were identified and quantified in the beetroot-cereal bar, namely, 3,4-dihydroxybenzoic, caffeic, chlorogenic, ferulic, gallic, p-coumaric, rosmarinic, syringic and vanillic acids. Gallic acid was the most abundant phenolic compound found in the product.
Data on phenolic compounds in several foods are not reliable, due to the lack of efficient methods that can efficiently extract phenolic acids. In many studies, phenolic composition is evaluated by using the Folin-Ciocalteau extraction method, which is simple and fast, but can exacerbate the results due to the interference of certain substances, such as ascorbic acid, present in food matrices. It also exhibits other disadvantages, such as low specificity and lack of identification, providing only estimates on the total phenolic compound content in samples [30]. In the present study, phenolic compounds were identified and quantified by HPLC, leading to high sensitivity, accuracy and repeatability, following an exhaustive extraction of both soluble and insoluble phenolic acids, since three different hydrolysis methods were applied to the same sample. In a previous assessment [31], 3,4-dihydroxybenzoic and chlorogenic acids were not detected in beetroot formulations and gallic, syringic, caffeic, ferulic acids and total phenolic compound contents were lower than described herein.
Furthermore, total phenolic compound concentrations in the beetroot-cereal bar (147.73 mg∙100 g-1 or 1477 mg∙kg-1) were higher than in juice prepared from the Beta vulgaris variety, rubra, ranging from 977.2±5.2 to 1450.3±42.1 mg∙L-1 [32]. Also, total phenolic compound concentrations in beetroot-cereal bar (147.73 mg∙100 g-1 or 1477 mg∙kg-1) were lower than results obtained from the Phenol-Explorer 3.6 database (http://phenol-explorer.eu), where an amount of 164.10 mg∙100 g-1 for Beta vulgaris rubra L. is described.
Antioxidant activity and lipid peroxidation inhibition
The beetroot-cereal bar presented 95.16±0.39% TAP, 78.46±0.95% of in vitro radical DPPH• formation, 21.19±1.29 mmol∙100 g-1 of in vitro radical ABTS• formation (TEAC), 10.63±1.38 mmol∙100 g-1 of FRAP and 9.45±1.02 mmol∙100 g-1 of ORAC (Figure 1a). The beetroot-cereal bar showed greater or similar antioxidant activities to synthetic antioxidants (BHT and BHA) and superior antioxidant activity compared to α-tocopherol. The effect of 20 mg∙mL-1 of each beetroot-cereal bar, BHT and BHA, and α-tocopherol on lipid peroxidation of a linoleic acid emulsion after 162 h was 85.7, 86.4, 76.3 and 62.9%, respectively (Figure 1b).
The antioxidant ability of beetroot-cereal bar may be related to several phenolic compounds found in these food matrices (Table 2). Phenolic compounds may present different physiological targets and action mechanisms [33]. Thus, it is difficult to choose a method able to accurately measure food antioxidant potential, due to the complexity of oxidative processes that may occur in food matrices or in biological systems.
Beetroot-cereal bars were formulated from beetroot juice and chips (concentrated beetroot in the powder form) and the concentration of beetroot bioactive nutrients increased in this form, as expected, confirming the highest antioxidant activity as determined by different analytical methods. The cereals and other components used in the beetroot-cereal bar formulation may also contribute to phenolic compound (Tables 2) and antioxidant (Figure 1a, b) activities. The results presented herein are in agreement with those reported by Wruss et al. [31], who demonstrated that antioxidant capacity is strongly dependent on the phenolic content of food matrices.
During the beetroot-cereal bar manufacturing process, the pre-cooked matrix was oven-dried by heat at temperatures above 100°C. For beetroot chip production, thin beetroot slices were also dried at the same temperature. Madrau et al. [34] demonstrated that hot air drying at mild temperatures, i.e., 55°C and 75°C, in two apricot cultivars increased phenolic content and antioxidant activity. This outcome can be explained by the breakdown of insoluble phenolic compounds, leading to better extractability of these larger compounds, perhaps generating novel bioactive phenolic compounds resulting from the heat and drying treatments [34].
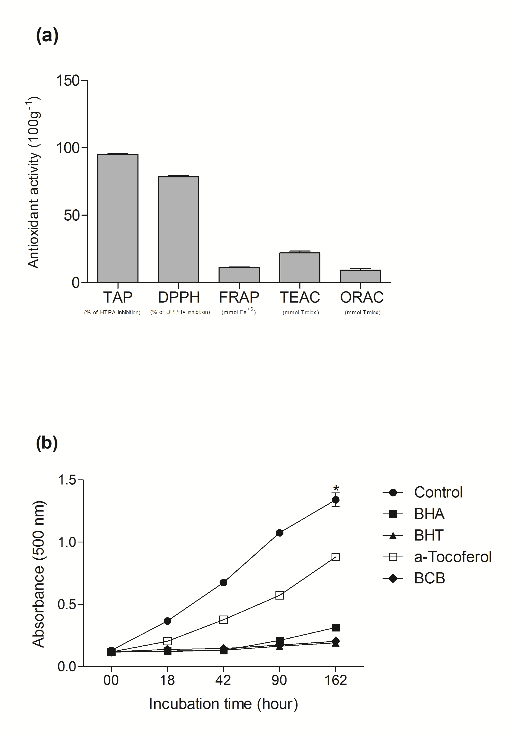
Figure 1. Total antioxidant potential (TAP), DPPH assays, FRAP, TEAC and ORAC assays of the beetroot-cereal bar were carried out (a). Values are expressed as means ± SD (n=3). The ability for beetroot-cereal bar to inhibit lipid peroxidation was assessed. Control H2O-DD, BHA (buthylated hydroxyanisole), BHT (butylated hydroxytoluene), α-tocopherol, beetroot-cereal bar. Samples were taken at the same concentration (20 mg∙ml-1) in a linoleic acid auto-oxidation system, followed during 162 h (b). Values are expressed as means ± SD (n=3). Different letters indicate differences between samples (two-way ANOVA, Bonferroni post hoc test; p <0.001). The symbol *(p <0.05) indicates differences between BHA, BHT and the beetroot-cereal bar (one-way ANOVA, Bonferroni post hoc test; p < 0.001).
Apparent color and texture
The color values for the beetroot-cereal bar are presented in Table 3. According to the CIE system scale ranging from 0 to 100, the beetroot-cereal bar showed high lightness (L*), red (a*) and yellowness (b*) color values.
Color affects the attractiveness of food products, interfering in sensory evaluations and purchasing decisions [11]. Herein, a color analysis was applied to evaluate any undesirable characteristics in the beetroot formulations. The red color was predominant in beetroot-cereal bar, since the tuberous root contains approximately 95% of betanin, a water-soluble heterocyclic compound that confers a purplish coloration to this vegetable [11].
The texture of the beetroot-cereal bar following the simulation of cutting by molar teeth during the first bite is presented in Table 3. The product presented a higher maximum force value, as this beetroot-derivative contains cereals and other natural binding agents that present greater crystallization capacity.
Texture was also evaluated in the beetroot-cereal bar, since firmness, spread ability and mouth feel, among other aspects, are important sensory and quality food indices. Yield stress was performed in beetroot-cereal bar to determine the strength of the coherent network structure throughout the volume of the material expressed as the force per unit area required to break down the structure [10]. The beetroot-cereal bar described herein showed reduced cut resistance when compared to others described previously [10].
| Table 3. Color and texture parameters of the beetroot-cereal bar | ||
|
Color parameters |
Beetroot-cereal bar |
|
|
L* |
26.91±3.35 |
|
|
a* |
9.25±1.98 |
|
|
b* |
5.96±2.12 |
|
|
Texture (NF) |
5.91±3.50 |
|
Values are expressed as means ± SD.
Shelf-life
The shelf-life of the beetroot-cereal bar was evaluated by testing the microbial quality of the bars for 30 days. Absence of contamination by yeasts, molds, Salmonella sp., E. coli, Bacillus cereus, and coliforms were confirmed up to 30 days from the processing of beetroot-cereal bar and under the limits considered safe established by the current Brazilian sanitary legislation for food for human consumption [35]. Taken together, the results of the microbiological analyses and shelf-life indicate that the processing was performed under satisfactory hygienic conditions established by the legislation.
Sensory analysis and intention to purchase
Table 4 indicates that the beetroot-cereal bar received relatively high mean scores in all sensory attributes. The overall acceptability received average scores corresponding to “really liked” (score 7.89), indicating a high product acceptance relation. Individual color, flavor, aroma and texture attributes presented scores between 6.9 and 7.5. In addition, the beetroot-cereal bar received higher mean scores regarding intent to purchase, corresponding to ‘probably would buy’.
The sensory evaluations demonstrated that the designed beetroot-cereal bar formulation displays an adequate overall acceptability for a well-designed food product considering its nutritional characteristics, antioxidant properties, NO3- concentration and dietary fibers. Furthermore, the beetroot-cereal bar could also be used to improve endothelial function with a consequent reduction of blood pressure in normal and hypertensive individuals with active or at high risk for cardiovascular diseases and improve cerebrovascular hemodynamics.
|
Table 4. Sensory analysis and purchase intent of the beetroot-cereal bar |
|
|
Sensory attributes |
Beetroot-cereal bar |
|
Color |
7.39±0.85 |
|
Aroma |
7.50±0.88 |
|
Flavor |
6.93±1.62 |
|
Texture |
7.52±1.34 |
|
Overall acceptability |
7.89±1.01 |
|
Purchase intention |
4.10±1.23 |
Values are expressed as means ± SD. Acceptance attributes (aroma, color, taste and overall acceptability) were assessed by applying a structured 9-point hedonic scale (1=dislike extremely, 9=like very much). Purchase intention was evaluated using a structured 5-point hedonic scale (1=would certainly not buy, 5=would certainly buy)
SBP, DBP and HR evaluation after chronic beetroot-cereal bar intake
SBP and DBP decreased after 3-weeks beetroot-cereal bar supplementation (Pre: 164.8±24.25 and 91.75±6.23 mm Hg; Post: 150.3±20.68 and 83.25±5.18 mm Hg) (Figure 2a, b). No changes were observed in HR after 3-weeks of beetroot-cereal bar supplementation (Figure 2c).
Adaily intake of 9.57±0.13 mmol of NO3- provided by a serving portion (60 g) of an individual beetroot-cereal bar for 3 weeks, caused an SBP decrease of ≈-14.0 mm Hg and DBP decrease of ≈-6.5 mm Hg, in accordance with previous studies [5], which considered that near 30% of the ingested NO3- is converted to NO2-, and then to NO, in the stomach, through the NO3--NO2-/NO pathway. It has been demonstrated that the formed NO crosses the endothelium and rapidly diffuses into smooth muscle cells of the blood vessels to activate the soluble guanylate cyclase enzyme that converts guanosine triphosphate to cyclic guanosine monophosphate, decreasing Ca2+ concentrations. The decreases in intracellular Ca2+ reduce its complexation with calmodulin, promoting a relaxation of vascular smooth muscle cells, leading to lower blood pressure that would improve endothelial functional adaptation [33].
The beetroot-cereal bar, besides being rich in NO3-, is also attractive in appearance, taste and smell, easily administered, adequate in size portion and format, and thus, can be used to improve endothelial function with consequent reduction of blood pressure in normal and hypertensive individuals with active or at high risk for cardiovascular diseases and improve cerebrovascular hemodynamics.
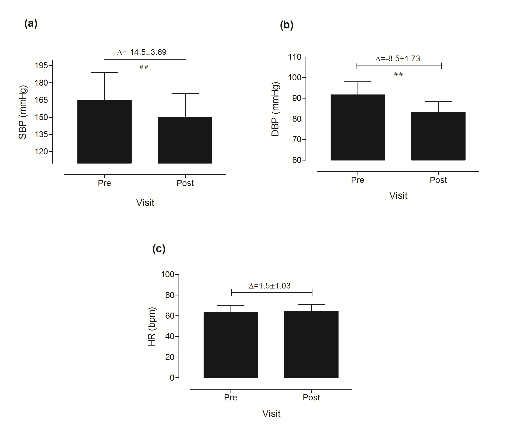
Figure 2. Effects of 3 weeks dietary NO3- supplementation (60 g of BCB – beetroot-cereal bar) on clinical SBP (systolic blood pressure) (a), clinical DBP (diastolic blood pressure) (b) and clinical HR (heart rate) (c). Data are expressed as means ± SD. The symbol ##(p <0.001) indicates difference between pre- and post-interventions.
The physico chemical and antioxidant characterization carried out herein, as well as the shelf-life stability and sensory analyses, indicate that the beetroot-cereal bar can be used as an adjuvant with vascular-protective effects in healthy, physically active individuals and in individuals who exhibit at least one CVD-related risk or those with established CVD. The results reported in this study encourage future studies concerning dietary NO3- supplementation by beetroot-cereal bar, including a careful evaluation of endothelial function and hemodynamic parameters in a randomized controlled crossover trial.
Supplementary file 1 Representative HPLC chromatogram of sugar (fructose, glucose, sucrose and maltose) standard analyses.
Supplementary file 2 Representative HPLC chromatogram of NO3- and NO2- standard analyses. NO2- was oxidized to NO3- by potassium permanganate (KMnO4-).
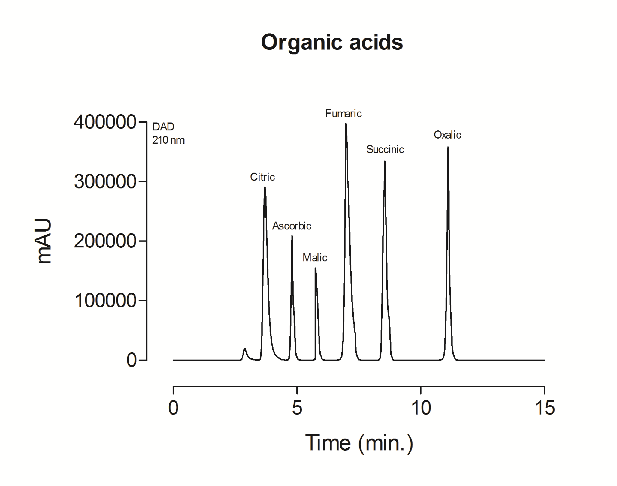
Supplementary file 3 Representative HPLC chromatogram of organic acid (citric, ascorbic, malic, fumaric, succinic and oxalic) standard analyses.
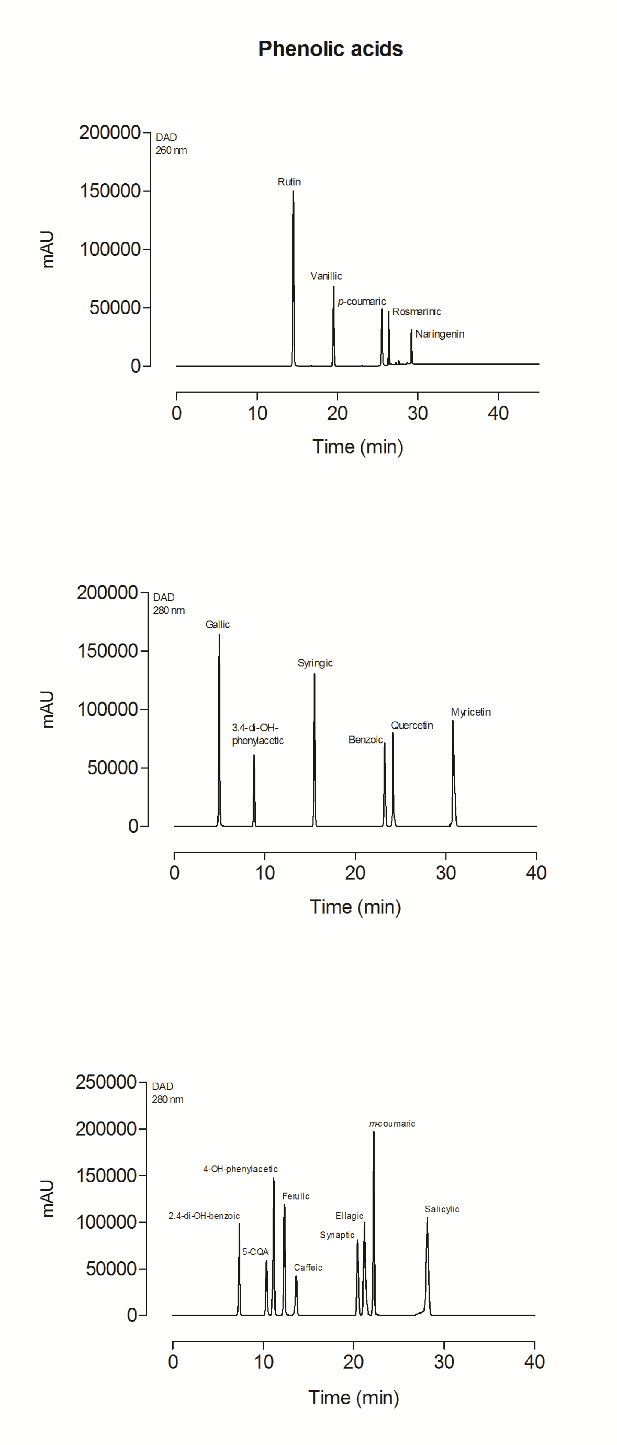
Supplementary file 4 Representative HPLC chromatogram of phenolic acid standard mixture.
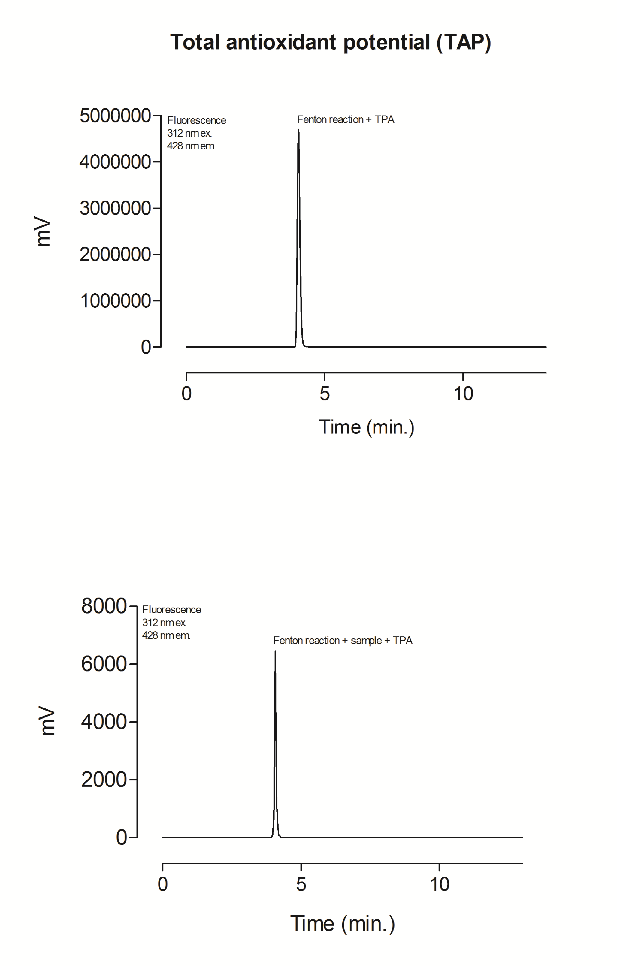
Supplementary file 5 Representative HPLC chromatogram of the products of a Fenton reaction with TPA and a Fenton reaction with samples and TPA.
AUTHOR CONTRIBUTIONS
Diego dos Santos Baião, Vânia Margaret Flosi Paschoalin and Eduardo Mere Del Aguila conceptualized and designed the research;
Diego dos Santos Baião, Fabrício de Oliveira Silva and Daniel Perrone analyzed the beetroot-cereal bar experiment data and interpreted the results;
Diego dos Santos Baião, Jenifer d’El-Rei and Mario Fritsch Neves analyzed the data and interpreted the results of the chronic beetroot-cereal bar supplementation;
Diego dos Santos Baião and Eduardo Mere Del Aguila prepared the figures and wrote the manuscript; Diego dos Santos Baião, Eduardo Mere Del Aguila, Vânia Margaret Flosi Paschoalin and Mario Fritsch Neves edited and revised the manuscript, and all authors critically revised the manuscript concerning important intellectual content, and read and approved the final manuscript.
ACKNOWLEDGEMENTS
The authors acknowledge financial support from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro). The authors thank Professor Lauro L.M.M. de Melo (UFRJ) for his help in data acquisition regarding consumer acceptance of the beetroot-cereal bar.
FUNDING
This research received a specific grant from the funding agencies CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, http://www.capes.gov.br) and FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, http://www.faperj.br).
World Health Organization/ Food and Agriculture Organization (2003) Diet, nutrition and the prevalence of chronic diseases. WHO/FAO Expert Consultation. Geneva (Technical Report 916). . Accessed 5 September 2018.
View ArticleDepartment of Health and Human Services, US Food and Drug Administration (2000) Food labeling: health claims; plant sterol/stanol esters and coronary heart disease. Interim final rule. Fed Regist 65(175): 54686-54739. PMid:11503640
PubMed/NCBIBaião DS, da Silva DVT, Del Aguila EM, Paschoalin VMF (2017) Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. Food Addit 2 (Chapter): 20-44.
View ArticleSingh B, Hathan BS (2017) Process optimization of spray drying of beetroot juice. J Food Sci Technol 54(8): 2241-2250. PMid:28740280 PMCid:PMC5502013
View Article PubMed/NCBIKapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A (2015) Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65(2): 320-327. PMid:25421976 PMCid:PMC4288952
View Article PubMed/NCBILidder S, Webb AJ (2013) Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br J Clin Pharmacol 75(3): 677-696. PMid:22882425 PMCid:PMC3575935
View Article PubMed/NCBIVasconcellos J, Conte-Junior CA, Silva DV, Pierucci APTR, Paschoalin VMF, Alvares TS (2016) Comparison of total antioxidant potential, and total phenolic, nitrate, sugar, and organic acid contents in beetroot juice, chips, powder, and cooked beetroot. Food Sci Biotechnol 25(1): 1-6.
View ArticleSun-Waterhouse D, Teoh A, Massarotto C, Wibisono R, Wadhwa S (2010) Comparative analysis of fruit-based functional snack bars. Food Chem 119(4): 1369-1379.
View ArticleBaião DS, Conte-Junior CA, Paschoalin VMF, Alvares TS (2016) Quantitative and comparative contents of nitrate and nitrite in Beta vulgaris L. by reversed-phase high-performance liquid chromatography-fluorescence. Food Anal Meth 9(4): 1002-1008.
View ArticleMattos MC, Galdeano MC, Carvalho CWP, Nogueira LC (2015) Efeito da adição de inulina e de sorbitol na textura de barras de cereais sem glúten. 5th Symposium on Food Security and Health. Bento Gonçalves. Food and health. SBCTA Regional, RS. . Accessed 15 September 2018.
View ArticleChandran J, Nisha P, Singhal RS, Pandit AB (2014) Degradation of colour in beetroot (Beta vulgaris L.): a kinetics study. J Food Sci Technol 51(10): 2678-2684. PMid:25328211 PMCid:PMC4190260
View Article PubMed/NCBIAssociation of Official Analytical Chemists (2000) Official methods of analysis. Rockville, WA.
Inada KOP, Oliveira AA, Revorêdo TB, Martins ABN, Lacerda ECQ, Freire AS, Braz BF, Santelli RE, Torres AG, Perrone D, Monteiro MC (2015) Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciariajaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J Funct Foods 17: 422-433.
View ArticleSilva FO, Perrone D (2015) Characterization and stability of bioactive compounds from soybean meal. LWT – Food Sci Technol 63(2): 992-1000.
Leite AM, Leite DC, Del Aguila EM, Alvares TS, Peixoto RS, Miguel MA, Silva JT, Paschoalin VM (2013) Microbiological and chemical characteristics of Brazilian kefir during fermentation and storage processes. J Dairy Sci 96(7): 4149-4159. PMid:23628252
View Article PubMed/NCBIda Silva DV, Silva FO, Perrone D, Pierucci APTR, Conte-Junior CA, Alvares TS, Del Aguila EM, Paschoalin VMF (2016) Physicochemical, nutritional, and sensory analyses of a nitrate-enriched beetroot gel and its effects on plasmatic nitric oxide and blood pressure. Food Nutr Res 60: 1-9. PMid:26790368 PMCid:PMC4720688
View Article PubMed/NCBIBenzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of ''antioxidant power'': the FRAP assay. Anal Biochem 239(1): 70-76. PMid:8660627
View Article PubMed/NCBIRe R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation descolorization assay. Free Radi Biol Med 26(9-10): 1231-1237. 00315-3
View ArticleZuleta A, Esteve MJ, Frígola A (2009) ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem 114(1): 310-316.
View Articlevon Gadow A, Joubert E, Hansmann CF (1997) Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathuslinearis), α-tocopherol, BHT, and BHA. J Agr Food Chem 45(3): 632-638.
View ArticleSamaranayaka AGP, Li-Chan ECY (2008) Pacific hake (Merluccius productus) fish protein hydrolysates with anti-oxidative properties. Food Chem 107: 768-776. . Accessed 01 September 2018. https://doi.org/10.1016/j.foodchem.2007.08.076
View ArticleAmerican Public Health Association (APHA) (2005) American water works association (AWWA) & water environment federation (WEF) Standard methods for the examination of water and wastewater. . Accessed 25 September 2018.
View ArticleMeilgaard MC, Carr T, Civille GV (2007) Sensory evaluation techniques. In: Taylor & Francis Group, an Informal Group Co (4th eds), Boca Raton, Florida, pp 464.
Brazil, National Health Surveillance Agency (2012) Technical regulation on complementary nutrition information. Ministry of Health, Resolution RDC N°54. . Accessed 20 September 2018.
View ArticleJones JM, Engleson J (2010) Whole grains: benefits and challenges. Annu Rev Food Sci Technol 1: 19-40. PMid:22129328
View Article PubMed/NCBIWalford G, Loscalzo J (2003) Nitric oxide in vascular biology. J Thromb Haemost 1(10): 2112-2118.
View ArticleBahadoran Z, Mirmiran P, Kabir A, Azizi F, Ghasemi A (2017) The nitrate-independent blood pressure-lowering effect of beetroot juice: a systematic review and meta-analysis. Adv Nutr 8(6): 830-838. PMid:29141968 PMCid:PMC5683004
View Article PubMed/NCBIMatsuura M (2001) Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr 131(3s): 1000S-1005S. PMid:11238805
View Article PubMed/NCBIPodolak RK, Zays ZE, Kastner CL, Fung DYC. Inhibition of Listeria monocytogenes and Escherichia coli 0157:H7 on beef by application of organic acids. J Food Protec. 1996; 59(4): 370–73.
View ArticleKhoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18(2): 2328-2375. PMid:23429347
View Article PubMed/NCBIWruss J, Waldenberger G, Huemer S, Uygun P, Lanzerstorfer P, Müller U, Höglinger O, Weghuber J (2015) Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J Food Composit Anal 42: 46-55.
View ArticleVáli L, Stefanovits-Bányai E, Szentmihályi K, Fébel K, Sárdi E, Lugasi A, Kocsis I, Blázovics A (2007) Liver-protecting effects of table beet (Beta vulgaris var. rubra) during ischemia-reperfusion. Nutrition 23(2): 172-178. PMid:17234508
View Article PubMed/NCBILiu YJ, Zhan J, Liu XL, Wang Y, Ji J, He QQ (2014) Dietary flavonoids intake and riskof type 2 diabetes: A meta-analysis of prospective cohort studies. Clin Nutr 33(1): 59-63. PMid:23591151
View Article PubMed/NCBIMadrau MA, Piscopo A, Sanguinetti AM, Del Caro A, Poiana M, Romeo FV, Piga A (2008) Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur Food Res Technol 228: 441-448.
View ArticleBrazil, National Health Surveillance Agency (2001) Technical regulation on microbiological standards for foods. Ministry of Health, Resolution RDC N°12. . Accessed 20 September 2018.
View Article