Dr Christos Kontogiorgis,
Tel: +302551030546, Fax: +30255130601, Email: ckontogi@med.duth.gr
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 4
Page No: 360-377
Dr Christos Kontogiorgis,
Tel: +302551030546, Fax: +30255130601, Email: ckontogi@med.duth.gr
Deligiannidou Georgia-Eirini 1, Kontogiorgis Christos2*, Hadjipavlou-Litina Dimitra3, Lazari Diamanto4, Konstantinidis Theodoros2 and Papadopoulos Athanasios1
1 Department of Nutrition and Dietetics, Faculty of Food Technology and Nutrition, Alexandrion Technological Educational Institution, 57400 Sindos, Thessaloniki, Greece
2 Laboratory of Hygiene and Environmental Protection, Democritus University of Thrace, Alexandroupolis, Greece
3 Department of Pharmaceutical Chemistry, School of Pharmacy, Faculty of Health Sciences, Aristotle University of Thessaloniki, 54124, Thessaloniki, Greece
4Department of Pharmacognosy-Pharmacology, School of Pharmacy, Faculty of Health Sciences Aristotle University of Thessaloniki, 54124, Thessaloniki, Greece
Yiannis Kourkoutas(ikourkou@mbg.duth.gr)
Mahendran Sekar(mahendransekar_05@yahoo.co.in)
Huey-Chun Huang(lchuang@mail.cmu.edu.tw)
Yoshinori Saito(saiyoshi@nagasaki-u.ac.jp)
Christos Kontogiorgis, ANTIOXIDANT CONTRIBUTION OF LAVENDER (Lavandula angustifolia), SAGE (Salvia officinalis), TILIA (Tilia tomentosa) AND SIDERITIS (Sideritis perfoliata) BEVERAGES PREPARED AT HOME.(2018)SDRP Journal of Food Science & Technology 3(4)
Background: Nature provides us with all the necessary ingredients to lead a healthy life, while data from ancient civilizations and scientific discoveries support the fact that the use of aromatic plants and herbs due to their therapeutic properties is a neglected healthy habit.
Methods: This study examines the antioxidant activity in domestic preparation of four herb beverages Lavender (Lavandula angustifolia), Sage (Salvia officinalis), Tilia (Tilia tomentosa) and Sideritis (Sideritis perfoliata) aiming to investigate the presence of antioxidants in the plants and to define which domestic preparation method of the beverage (decoction/infusion) ensures better antioxidant capacity. In the experimental procedure, herbal decoctions and infusions (boiling time 5 minutes) were extracted with 4 different solvents of increasing polarity (petroleum ether, diaithylether, ethyl acetate, butanol). This process resulted to 10 samples from each herb beverage. Each of them was examined for the TPC via the Folin - Ciocalteau method, the interaction with the cationic radical ABTS, the free stable radical DPPH for the determination of antioxidant capacity, the hydroxyl radical scavenging ability, the inhibition of lipid peroxidation of linoleic acid and finally the acceptability of each beverage with respect to the manner of preparation through sensory evaluation in a total number of 40 random volunteers.
Results: Τhe results exhibit the characteristics represented by each herb. Comparison and classification between them was also performed. The antioxidant characteristics of the herbs were affected both by the preparation method as well as the extraction solvent.
Conclusions: All herbs exhibited high antioxidant capacity. However, not all were positively evaluated during the organoleptic evaluation which raises the question of whether these beneficial herbs would be included as beverages in every-day diet. Our research exhibited the capacity of these herbs as well as the need for organoleptic evaluation to be included in research.
Abbreviations: TPC: Total Phenolic Content, TCM: Traditional Chinese Medicine, TEAC: Trolox-Equivalent Antioxidant Capacity, OD: Optical Density, GAE: Gallic Acid Equivalent, HAT: Hydrogen Atom Transfer
Key words: Beverages, Antioxidants, Lipid Peroxidation, Phenolic Content, Lipoxygenase
It is widely known that many natural products are highly beneficial for good health. Ever since ancient times, and much prior to scientific thinking and verification, humans would use plant-based remedies for treatment and prophylaxis.[1] Mediterranean diet is associated with the prevention and treatment of chronic diseases and longevity, by also being a standard way of lifestyle. Beverages from herbs of Mediterranean, have an important role in the Mediterranean diet pyramid, both nutritionally and culturally.
Studies have shown that following the Mediterranean diet(-s) can lead to a 14% lower mortality rate, while there are numerous studies to support this beneficial diet in the framework of health promoting and disease prevention or treatment.[2–4] Additionally, Mediterranean-style diets are close to American Heart Association dietary guidelines.[5] Plant food bioactive compounds have been related to beneficial effects for a number of health conditions.[6–8] It is known that the Mediterranean diet’s composition contains large amounts of antioxidant vitamins (vitamins C, A, E and beta-carotene) that have a beneficial effect in the treatment of various diseases, such as those of the cardiovascular system, in type 2 diabetes, cancer and obesity while there are also findings that it can even reduce the risk of Alzheimer's and Parkinson's.[9–11]
As the base of the Mediterranean diet, fruits, vegetables, grains, seeds etc. have been the centre of attention for researchers worldwide. Although many spices and herbs, rich in flavonoids, have been used for years in medicine, the pharmaceutical usage of plant compounds had faced its ups and downs over the evolution of medicine, with a 2008 estimate stating that 25% of the commonly used medicines contain compounds isolated from plants.[12] However, the search for new molecules, has taken a route where the science of ethnobotany and ethno-pharmacognosy are being used as guide to lead the chemist towards different sources and classes of compounds.[13]
Flavonoids are now known for their antioxidative activity, free radical scavenging capacity, coronary heart disease prevention, hepatoprotective, anti-inflammatory, and anticancer activities, while some flavonoids exhibit potential antiviral activities.[14–18] Fruits and vegetables are the main dietary sources of flavonoids for humans, along with tea and wine. However, a clear understanding of the mechanisms of action of flavonoids, either as antioxidants or modulators of cell signalling, and the influence of their metabolism on these properties are key to the evaluation of these potent biomolecules as anticancer agents, cardio-protectants, and inhibitors of neurodegeneration.[19]
Although information on the working mechanisms of flavonoids is still not understood properly, increased flavonoid consumption is associated with reduced mortality from coronary heart disease in women and men and with reduced risk of coronary heart disease by 38% in postmenopausal women.[20–23] It seems that the combination of food and biological interactions of the different components of the Mediterranean diet provides important health benefits, with high antioxidant content, contributing significantly to the antioxidant value of this diet model.[9, 24–28]
The world of plants includes some 350,000 species, with 18,000 classified as aromatic and many classified as therapeutic due to substances they contain with therapeutic properties. Most herbs grown in Greece belong to the family of Labiatae (Lamiaceae).[29–35]
Lavender (Lavandula angustifolia) was used in ancient Rome and North Africa for perfuming and in TCM, in order to treat infertility, infection and stress, while in Arabic medicine it was used to treat stomach pain and kidney problems. It was widely used as an aphrodisiac during the Victorian era while later it has been classified as antidepressant, antispasmodic, anti-flatulence antiemetic, diuretic and tonic.[29,36–40] Its great appeal and commercial value was confirmed again when it was named Herb of the Year in 1999 by Herb Growing and Marketing Network in the United States of America.[16-17]
To date, Lavender (Lavandula angustifolia)continues to excite the scientific community through significant results both on in vitro testing and in clinical trials.[41–44]
Sideritis (Sideritis perfoliata), widely known as “mountain tea”, has been used for centuries as a home-made beverage due its soothing characteristics. It is widely used as aromatic and warming drink, for its anti-inflammatory properties. It is beneficial to the blood vessels of the heart and also has gastroprotective properties and is used in rheumatic diseases, diarrhea and dyspepsia.[38,45–48] Aside of its antioxidant characteristics, Sideritis has also been intensely researched for its effects on glucose and lipid disorders, while literature also mentions an interest on psychopharmacological effects.[49–52]
Tilia (Tilia tomentosa) has been known for their analgesic, antibacterial and anti-infective characteristics. Furthermore, studies have shown that Tilia can contribute as antispasmodic, antiviral and also auxiliary the circulatory system, while literature also mentions efficacy as adjuncts to adaptogens.[53–56]
Sage (Salvia officinalis) has been traditionally used to treat sweating and menopausal hot flushes, as well as to alleviate associated menopausal symptoms and as a general tonic, while in folk medicine, it has been used for the treatment of different kinds of disorders including seizure, ulcers, gout, rheumatism, inflammation, dizziness, tremor, paralysis, diarrhea, and hyperglycaemia.[57-58] Additionally, S. Lavandula efolia Vahl. (Spanish sage) extracts and constituents have demonstrated anticholinesterase, antioxidant, anti-inflammatory, oestrogenic and CNS depressant (sedative) effects all of which are relevant to the treatment of Alzheimer's disease (AD).[59]
Several studies aimed to the determination of the antioxidant activity of these herbs using a variety of methods and they conclude that herb extracts show strong antioxidant activity.[36-37,56,60–64] Furthermore, the impact of boiling conditions has often been examined, showing that the preparation conditions of a beverage influence its characteristics.[30,65–69]
This study examines the antioxidant capacity of beverages of Lavender (Lavandula angustifolia), Sage (Salvia officinalis), Tilia (Tilia tomentosa) and Sideritis (Sideritis perfoliata) and aims to point out the influence of preparation method in household conditions, on the antioxidant capacity. According to previous studies on plant extracts, there is a relation between the solvents’ polarity and free radical scavenging activity also related to the presence of phenolic acids, although a lot of research is related to the essential oils of the plants.[37, 70–72] In this research, we examine this aspect but also attempt a comparison among those four beverages considering their antioxidant characteristics examining them separately in vitro.
For the experimental procedure, we used dried herbs. Namely: Lavender (flowers), Sage (leaves) and Tilia (leaves and flowers) were procured from Myrtis nurseries which were collected and dried in Polykarpi Pozar Greece. Sideritis (all parts) was collected and dried in Pertouli Trikala Greece (packs of 50 gr).
Solvents and Solutions: Petroleum ether (PE), Diethyl ether (DE), Ethyl acetate (EA), 1-butanol (BuOH), 1,1-Diphenyl 2-picrylhydrazyl (DPPH), Folin-Ciocalteu reagent, 2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), 2,2’-azobis-(2-amidinopropane)-dihydrochloride (AAPH), Dimethyl sulfoxide (DMSO), Ethylenediaminetetraacetic acid (EDTA), lipoxygenase type I-B (soybean) and linoleic acid (sodium salt), 99% purity, were purchased from Sigma (St Louis, MO, USA). All chemical reagents were of analytical grade. Nash solution was obtained by adding 45g ammonium acetate, 0.9 ml acetic acid and 0.6 ml acetyl acetone in 100ml H2O. Regarding the preparation methods of the beverages, the boiling time was 5 minutes, in order to prepare the infusions and decoctions from each herb. Specifically, for the preparation of the infusion, the water was boiled for 5 minutes and then added with the 5g of dried herb on the contrary to the decoction where water and 5g of dried herb boil outset together for 5 minutes. In both cases the solution is filtered so as to separate the solid residue, which were subjected to extraction with four different solvents of increasing polarity.
2.1. Beverage’s Preparation
Distinguishing the difference between Infusion and Decoction. According to Merriam-Webster Infusion is a drink made by material using liquid (most commonly hot water) following these steps: (1) the liquid (usually water) was boiled (2) the material is added for a certain period under stirring and (3) the liquid extract was filtered. (Scheme 1)
Decoction is a different procedure following these steps: (1) the material was boiled in parallel with the liquid (usually water) and (2) the extract was filtered (Scheme 1). [73-74]
In order to be clear regarding the preparation method, as its significance would influence the outcomes of this study as described in the introduction, we have developed a schematic presentation of the process, shown in Scheme 1.
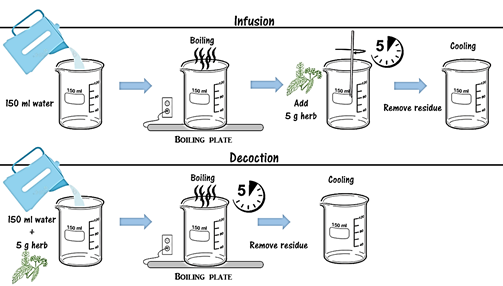
Scheme 1, Distinguishing the difference between Infusion and Decoction.
For the preparation of both procedures 5g of each herb were used in 150ml of distilled water and they left boiling on a hearth for 5 minutes. After 5 minutes, solid residue is removed and the mixture is left to cool.
Each part of the preparation took place separately for each herb following the exact same process. The mixture resulting from each process is subjected to extraction using solvents of increasing polarity: Petroleum ether (PE), Diethyl ether (DE), Ethyl acetate (EA) and 1-Butanol (BuOH). Each extract is evaporated, dried and the solid residue stored in the fridge. The whole procedure is repeated three times for each herb and the solid residues are stored in vials. We ultimately received overall 10 vials of solid residues, whose weights were measured in grams on an analytical balance and preserved sealed at 4°C for further analysis.[73]
2.2. Determination of Total Phenol Content
The total phenol content of the obtained fractions was determined using the method of Zheng and Wang with a few modifications: 1 mg of dry residue was dissolved in 1ml DMSO or H2O.[75] 10μl of this solution was transferred to a volumetric flask to which 50μl of Folin-Ciocalteu phenol reagent and 150μl of 20% sodium carbonate solution were added. The mixture was shaken thoroughly and kept for 120 minutes at 25-30oC, in absence of light. The absorbance of the blue color formed was measured at 765nm. The concentration of total phenol compounds for each extract was calculated on the basis of a standard curve obtained using gallic acid as the standard (twelve serial- 2-fold dilutions to give a range of 0.01– 0.001mg/mL in triplicate). Results were expressed as concentration of phenolic mg/L of each solvent compared to the different boiling times.
2.3. Antioxidant capacity
2.3.1. Evaluation of TEAC by ABTS
A method for the screening of antioxidant activity is reported as a decolorization assay applicable to both lipophilic and hydrophilic antioxidants, including flavonoids, hydroxycinnamates, carotenoids, and plasma antioxidants. The pre-formed radical mono cation of 2,2'-azinobis-(3- ethylbenzothiazoline-6-sulfonic acid) (ABTS+) is generated by oxidation of ABTS with potassium persulfate and is reduced in the presence of such hydrogen-donating antioxidants.[76] The Trolox Equivalent Antioxidant Capacity (TEAC) assay is based on the scavenging of the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical converting it into a colorless product. The degree of decolorization induced by a compound is related to that induced by Trolox, giving the TEAC value.[77]
In this assay, ABTS is converted to its radical cation by addition of sodium persulfate. This radical cation is blue in color and absorbs light at 734nm. During this reaction, the blue ABTS radical cation is converted back to its colorless neutral form. The reaction was monitored spectrophotometrically. For each sample, we prepared 3 mixtures which contained a 50μl sample -from the stock solution of the sample (approximately 1 mg in 1 ml DMSO/H2O and 1950μl ABTS (7mM). The mixture was shaken thoroughly and kept for 6 minutes at room temperature before measuring the absorbance.[21,76,78–81]
2.3.2. Interaction with Stable Free Radical of DPPH
DPPH assay test is very useful in the micromolar range. 1,1-Diphenyl-picrylhydrazyl is a stable free radical and antioxidants react with it and reduce it to DPPH-H while the absorbance decreases. The radical’s solution is purple color which turns yellow when the radical is scavenged, while the degree of discoloration indicates the scavenging potential of the antioxidant compounds or extracts in terms of hydrogen donating ability and can be followed spectrophotometrically (517nm).[21,32,64,81–83]
A 20μl sample from the stock solution of the sample (2.5mg/1ml DMSO) were dissolved in absolute ethanol to a final volume of 1 ml and then added to 1 ml DPPH (0.1mM, in absolute ethanol). The reaction mixture was kept at room temperature. The OD of the solution was measured at 517nm, after 20 minutes and 60 minutes. The optical densities of the samples in the absence of DPPH were subtracted from the corresponding OD with DPPH. The % reduction values were determined and compared to appropriate standards.
% Reduction = (control OD(mean)-sample OD(mean))/(control OD(mean))*100
2.3.3. Competition of the tested compounds with DMSO for hydroxyl radicals
The hydroxyl radicals generated by the FeCl3/ascorbic acid system, were detected by the determination of formaldehyde produced from the oxidation of DMSO. The reaction mixture contained EDTA (200μl), FeCl3 (150μl), DMSO (200μl) in phosphate buffer (240μl, pH 7.4), the tested compounds (10μl) and ascorbic acid (150μl). After 30 minutes of incubation (37oC) the reaction was stopped with the addition of CCl3COOH (17.5% w/v) and 1ml of Nash solution was inserted. After 10 minutes of incubation (60oC) the absorbance of the samples was measured at 734nm.[84–86]
2.3.4. Inhibition of Linoleic Acid Lipid Peroxidation
The water soluble azo compound AAPH is used as a free radical initiator for in vitro studies of free radical production. Production of conjugated diene hydroperoxide by oxidation of linoleic acid sodium salt in an aqueous solution is monitored at 234nm. An amount of 10μl of the 16mM linoleic acid sodium salt solution was added to the UV cuvette containing 0.93ml of 0.05M phosphate buffer, pH 7.4, pre-thermostated at 37oC. The oxidation reaction was initiated at 37oC under air by the addition of 50μl of 40mM AAPH solution. Oxidation was carried out in the presence of aliquots (10μl) in the assay without antioxidant, and lipid oxidation was measured in the presence of the same level of DMSO. The rate of oxidation at 37oC was monitored by recording the increase in absorption at 234 nm caused by conjugated diene hydroperoxides.[73,75,87-88]
2.3.5. Organoleptic Evaluation
Much of the potential of sensory information for understanding primate feeding has been ignored because the subject is usually approached from a nutritional perspective rather than a sensory one.[89]
Organoleptic properties are the aspects of food, water or other substances; that a person experiences via the senses-including taste, sight, smell, and touch. The organoleptic properties of food have a determining effect on consumption and commercial success. There are four major types of organoleptic testing: (1) Difference testing, which identifies differences from one sample to another, (2) Sensitivity testing, which assesses stimulus-response dynamics, (3) Preference testing, which involves ‘like’ or ‘dislike’ feedback in relation to the sample, (4) Descriptive testing, which dwells on rated characteristics.[90–94] There is limited information on the effect of the compounds of a beverage on its sensory characteristics and research usually focuses on its quality on maters other than acceptability.[90,95-96]
Due to lack of extent literature on the questionnaire requirements a questionnaire was created by making some adjustments to questionnaires used in previous studies.[90,94,96] The testing was descriptive, with mono-polar and bipolar scales for each required characteristic. Samples of each herb beverage were prepared at the same time (both decoction and infusion) and given to the testers at the same time after cooling. Before the evaluation, the questionnaire was explained to the panellists and each herb beverage was evaluated at a different time and each time from different panellists so as to avoid partial comparison in between herbs.
3.1. Isolation of organic extracts
After the preparation of each herb beverage, samples were extracted with a series of organic solvents with increasing polarity. Solid residues’ weight after the extraction and evaporation are shown in Table I. The results suggest that the solvents’ polarity influenced the residues weight with an increasing tendency, while there are not significant differences regarding the preparation method.
Table I: Solid Residue (g) after the Extractions
|
|
SOLVENTS |
PE |
DE |
EA |
BuOH |
H2O |
|
Solid residue (g) |
Lavender Infusion |
0.0480 |
0.0658 |
0.0760 |
0.2165 |
1.4945 |
|
Lavender Decoction |
0.0564 |
0.0622 |
0.0641 |
0.1401 |
1.9798 |
|
|
Sideritis Infusion |
0.1729 |
0.1834 |
0.2918 |
0.2134 |
1.7236 |
|
|
Sideritis Decoction |
0.1548 |
0.1739 |
0.1997 |
0.2458 |
1.8931 |
|
|
Tilia Infusion |
0.0357 |
0.0383 |
0.0391 |
0.1627 |
1.0827 |
|
|
Tilia Decoction |
0.0166 |
0.0211 |
0.0212 |
0.2878 |
1.0640 |
|
|
Sage Infusion |
0.3228 |
0.0526 |
0.0732 |
0.1054 |
1.1046 |
|
|
Sage Decoction |
0.5020 |
0.1012 |
0.4223 |
0.0715 |
1.0697 |
In all cases the solvents polarity had an effect on the residue’s quantity, favouring the most polar solvents in most cases except the case of Sage in which resulted to an abnormal distribution between solvents in the decoction samples. Lastly, in all cases the decoction extracts performed better in the residue giving part, which gave us the first hint on the debate of the best preparation method of the beverage.
3.2 Determination of TPC
Phenolic compounds are widely distributed in plant kingdom and they present significant antioxidant capacity due to their ability to donate hydrogen and form stable radical intermediates. Non-structural phenolic compounds perform a variety of functions in plants, including acting as antioxidants.[97] Phenolic antioxidants are products of secondary metabolism in plants, and their antioxidant activity is mainly due to their redox properties and chemical structure, which can play an important role in chelating transitional metals, inhibiting enzymes and scavenging free radicals. [98–100]
In this assay, the amounts of total phenolic content in the extracts was determined spectrophotometrically using Folin-Ciocalteu reagent and calculated as GAE. The Folin-Ciocalteu assay relies on the transfer of electrons in alkaline medium from phenolic compounds to phosphomolybdic/phosphotungstic acid complexes, which are determined spectroscopically at 765 nm.
Results, as shown in Table II, indicate an increasing tendency of the phenolic content related to the extract solvent’s polarity, while for most extracts, mid-polar solvents exhibit higher phenolic content. Lavender and Tilia extracts had significant increase of TPC related to the increased polarity of the solvent of 74,67% and 89.90%, respectively. On the contrary, although exhibiting higher average rates of TPC related to the other herbs, Sage and Sideritis rates of TPC related to the increased solvent’s polarity, was reduced in a minimum level for Sage, however dramatically for Sideritis (over 60% reduction).
Regarding the preparation method, decoction samples had higher average results of phenolic content for 3 out of 4 herbs tested, while in the case of Sideritis, decoction samples showed a lesser reduction with regards to the solvent’s increasing polarity.
Table II: Total Phenolic Content (mg/l) Pivot Table
|
|
SOLVENTS |
PE |
DE |
EA |
BuOH |
H2O |
|
Total Phenolic Content (mg/l) |
Lavender Infusion |
35.232 |
55.224 |
90.739 |
104.073 |
106.073 |
|
Lavender Decoction |
22.768 |
72.333 |
99.289 |
105.130 |
130.594 |
|
|
Sideritis Infusion |
99.127 |
44.233 |
46.722 |
33.763 |
38.874 |
|
|
Sideritis Decoction |
46.861 |
51.426 |
29.574 |
37.066 |
28.553 |
|
|
Tilia Infusion |
4.436 |
105.089 |
280.696 |
142.752 |
56.205 |
|
|
Tilia Decoction |
7.193 |
228.626 |
277.444 |
83.626 |
58.478 |
|
|
Sage Infusion |
46.378 |
83.255 |
173.118 |
184.489 |
63.732 |
|
|
Sage Decoction |
79.647 |
134.772 |
148.217 |
194.896 |
60.647 |
3.3 Assay of radical cation scavenging activity
The radical cation of ABTS is used for classification of relative inhibition capacity of flavonoids and phenolic compounds through their ability as electron or proton donors.
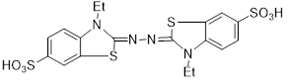
Scheme 2: Structure of f 2,2′-azinobis (3- ethylbenzothiazoline-6-sulfonicacid) (ABTS+)
When the radical interacts with antioxidants its concentration is reduced, recorded as absorption decrease. Thus, the higher the antioxidant activity of the tested substances the lower the absorption is since antioxidants interact with the colored cation in proportion to their concentration in each sample. The absorption maxima (λmax) of ABTS+ adopted by most investigators to spectrophotometrically monitored the reaction between the antioxidant and ABTS are 415 nm and 734 nm.
In this assay, ABTS is oxidized to its radical cation, ABTS+. (Scheme 2), which is intensely colored. Antioxidant capacity is measured as the ability of test compounds to decrease the color reacting directly with the ABTS+. radical. The results are expressed as Trolox equivalents and the measurements were taken after 6 minutes at 734 nm.
Table III: Antioxidant capacity of herbs’ infusion and decoction preparation, via ABTS method (μM Trolox)
|
|
SOLVENT |
PE |
DE |
EA |
BuOH |
H2O |
|
Antioxidant capacity (μM Trolox) |
Lavender Infusion |
317.148 |
666.517 |
778.396 |
870.179 |
719.365 |
|
Lavender Decoction |
304.008 |
769.673 |
951.600 |
801.766 |
752.439 |
|
|
Sideritis Infusion |
129.027 |
346.062 |
858.623 |
780.610 |
466.019 |
|
|
Sideritis Decoction |
152.900 |
250.566 |
917.089 |
608.426 |
283.022 |
|
|
Tilia Infusion |
176.200 |
3777.17 |
8286.51 |
6,422.07 |
1346.65 |
|
|
Tilia Decoction |
303.280 |
4480.24 |
8118.59 |
2797.59 |
2148.59 |
|
|
Sage Infusion |
1074.72 |
2459.43 |
3440.95 |
7126.08 |
1046.47 |
|
|
Sage Decoction |
3496.67 |
33632.08 |
3786.13 |
30691,96 |
2752.89 |
The results (Table III) exhibit higher rates on extracts of mid-polar solvents in most cases regardless of the herb or the preparation method, while on the preparation method, the findings show that decoctions of each beverage presented higher average rates in 3 out of 4 cases and an overall rate of 42% higher than infusions. Out of a total of 40 samples, only 10 were above the average rates in this assay, 5 in the Sage group and 5 in the Tilia group, while 6 were decoctions and 4 infusions. Overall 10 lowest rates were observed in Lavender, Sideritis and Tilia in a distribution 2:6:2, while 6 of these rates were observed in Petroleum ether (PE) extracts, 2 in Diethyl ether (DE) extracts and only 2 in water extracts.
3.4 Interaction with Stable Free Radical of DPPH
Antioxidant compounds like phenolic acids, polyphenols and flavonoids scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl inhibiting the oxidative mechanisms that lead to degenerative diseases.
The DPPH assay is widely used in plant biochemistry to evaluate the properties of plant constituents for scavenging free radicals. Several protocols are known considering DPPH assay; using different conditions such as different reaction times, solvents, pH taking different antioxidant standards.[21,82,101] The protocol followed in the present study, includes Trolox as the reference standard and two times of measurement of the extracts (20 minutes and 60 minutes).
According to our results, all the extracts interacted with the stable free radical DPPH and in most cases the reaction was over 50% regardless of the time of the measurement (20 minutes or 60 minutes), the preparation method or the solvent of the extract, as shown in Figure 1 and Figure 2. In the measurement of 20 minutes, only 17 samples rated less than 85% interaction, while during the 60 minutes measurement only one sample managed to pass the threshold of 50%. Among the lowest rates (15 samples with less than 50% reaction), 10 were observed in in Petroleum ether (PE) extracts, 4 of which were decoctions. Over the 60 minutes measurements, the overall reaction rate was 78.07%, with only 11 samples rating lower than average. None of the samples in the Sideritis group exhibited reaction less than 65%.
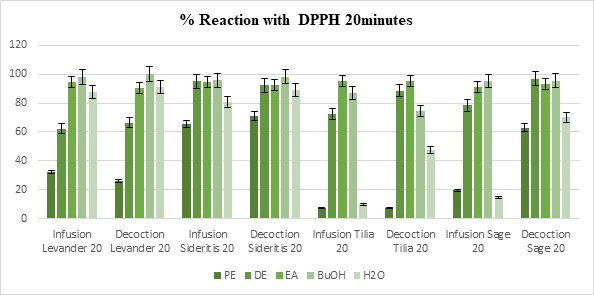
Figure 1: % Reaction of the extracts with DPPH standard radical at 20 minutes time. Each value represents the mean of three independent measurements in each sample (The results were averaged, and the standard deviation of absorbance was less than 10% of the mean). Statistical studies were done with student’s T-test, p < 0.05
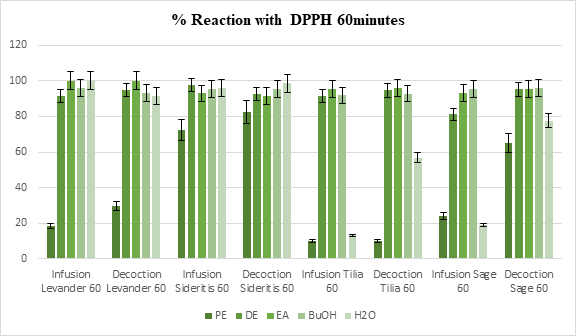
Figure 2: % Reaction of the extracts with DPPH standard radical at 60 minutes time. Each value represents the mean of three independent measurements in each sample (The results were averaged, and the standard deviation of absorbance was less than 10% of the mean). Statistical studies were done with student’s T-test, p < 0.05
Considering the polarity of the solvents used, polar extracts show higher free radical scavenging activity than non-polar ones and this can be related to the presence of phenolic acids and flavonoids. The average reaction in the water extracts of all samples was 65.2% and Petroleum ether (PE) extracts averaged at 37.8%, while the average rate of the solvents in between was 91.7%.
3.5. Competition of the tested extracts with DMSO for hydroxyl radicals
Oxidative stress—the production and accumulation of reduced oxygen intermediates such as superoxide radicals, singlet oxygen, hydrogen peroxide, and hydroxyl radicals—can damage lipids, proteins, and DNA.[102] The hydroxyl radical (OH) is one of the most powerful oxidizing agents, able to react unselectively and instantaneously with the surrounding chemicals, including organic pollutants and inhibitors.[103] Hydroxyl radicals are among the most reactive oxygen species and are considered to be responsible for some of the tissue damage occurring in inflammation. DMSO is a compound, which readily reacts with hydroxyl radical and it strongly suppresses hydrogen peroxide.[98,104].
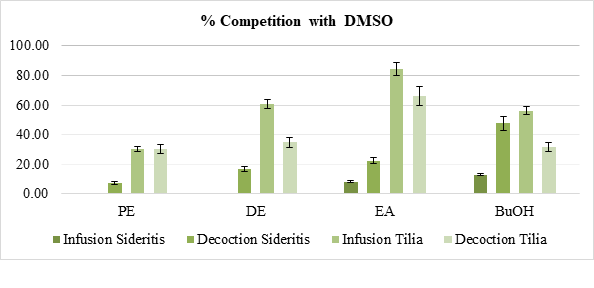
Figure 3: Competition of the tested extracts of Sideritis and Tilia beverages (Infusion and Decoction Preparation) with DMSO for hydroxyl radicals. Each value represents the mean of three independent measurements in each sample (The results were averaged, and the standard deviation of absorbance was less than 10% of the mean). Statistical studies were done with student’s T-test, p < 0.05.
Regarding the hydroxyl scavenging ability of the tested extracts from Sideritis - (Figure 3) an increasing tendency was observed depending on the solvent polarity. However not any pick was recorded in the aqueous samples. Similar increasing tendency occurs in Tilia samples. Overall, only 4 samples resulted with average rates higher than 50%, all of which were in the Tilia group, 3 of which were infusions.
3.6. % Inhibition of Linoleic Acid Lipid Peroxidation
AAPH was used as a free radical initiator to follow oxidative changes of linoleic acid to conjugated diene hydroperoxide. Azo compounds generating free radicals through spontaneous thermal decomposition are useful for free radical production studies in vitro. The water-soluble azo compound AAPH has been used as a clean and controllable source of thermally produced alkyl peroxyl free radicals. In the AAPH assay, the highly reactive alkyl peroxyl radicals are intercepted mainly by HAT from the antioxidant.[101,105,106]
In this experiment, the ability of Sage and Tilia extracts to inhibit the peroxidation of linoleic acid was in question. These two herbs have been previously tested for their antioxidant ability separately or in contrast to each other and there are literature findings supporting non-significant differences in their capacity. [54–56]
Having found differences between them, further analysis of the antioxidant capacity as shown in the previous experiments further study was suggested. The samples of both beverages were tested at two different times, one as concentrated sample (Table V (Sage) and (Tilia)) and then as a diluted sample (1:10 dilution) in order to estimate their inhibiting ability in a lower concentration. All samples presented inhibition with an average of 75% for the infusion treatment of preparation and 83% for the decoction.
Table IV 4: % Inhibition of lipid peroxidation of linoleic acid by Sage and Tilia beverage (Infusion and Decoction Preparation)
|
PE |
DE |
EA |
BuOH |
H2O |
|
|
Infusion Sage |
45 ± 3.5 |
85.6 ± 6.1 |
100 ± 9.1 |
100 ± 7.8 |
45 ± 3.9 |
|
Decoction Sage |
95.8 ± 7.7 |
88.5 ± 8.2 |
92.3 ± 6.3 |
100 ± 8.2 |
41.6 ± 3.8 |
|
Infusion Tilia |
39.6 ± 4.1 |
89.8 ± 8.5 |
82.8 ± 7.5 |
74.5 ± 6.3 |
8.4 ± 7.1 |
|
Decoction Tilia |
26.7 ± 2.9 |
100.0 ± 8.8 |
96.1 ± 5.2 |
51.2 ± 4.1 |
6.5 ± 0.8 |
Regarding the diluted samples (Table V), a rapid reduction of the % inhibition ability was observed in both herbs extracts. An average of 47.6% for the infusion of Sage and 61.6% for the decoction was estimated. Tilia diluted samples had an average decrease of 41.6% for the infusion and 31.1 % for the decoction treatment compared to the concentrated samples.
Table V: % Inhibition of lipid peroxidation of linoleic acid by Sage and Tilia beverage (Infusion and Decoction Preparation Diluted Samples 1:10)
|
|
PE |
DE |
EA |
BuOH |
H2O |
|
Infusion Sage |
87.1 ± 7.6 |
35.5 ± 3.3 |
46.6 ± 4.2 |
54.4 ± 4.2 |
3.5 ± 0.4 |
|
Decoction Sage |
25.6 ± 2.9 |
30.9 ± 2.2 |
28.7 ± 2.1 |
24.6 ± 2.1 |
0.4 ± 0.0 |
|
Infusion Tilia |
13.8 ± 0.8 |
23.2 ± 1.9 |
18.4 ± 1.6 |
25.1 ± 2.1 |
6.6 ± 0.6 |
|
Decoction Tilia |
23.7 ± 2.5 |
26.4 ± 1.8 |
40.5 ± 3.9 |
27.8 ± 2.6 |
6.7 ± 0.4 |
The overall average decreased from 68.47% (original samples) to 27.47% (diluted samples). Among the original samples, 12 were above average rates, 11 of which were observed in mid-polarity solvents, while among the diluted samples, only 8 were above average rates, 7 of which were observed in mid-polarity solvents.
3.7. Organoleptic Evaluation
The primary goal of the organoleptic evaluation was to get a feedback on whether a beverage of these herbs is acceptable for consumption with respect to the preparation method without adding any sweeteners.
There has been an evaluation of various characteristics such as turbidity, color, bitterness, acidity using a unipolar scale in a questionnaire (Results not shown) as well as flavor, taste and liking using a bipolar scale (Scheme3), it was considered important to present only the results of the liking of each beverage and discuss the rest of our findings in reference to other organoleptic characteristics.
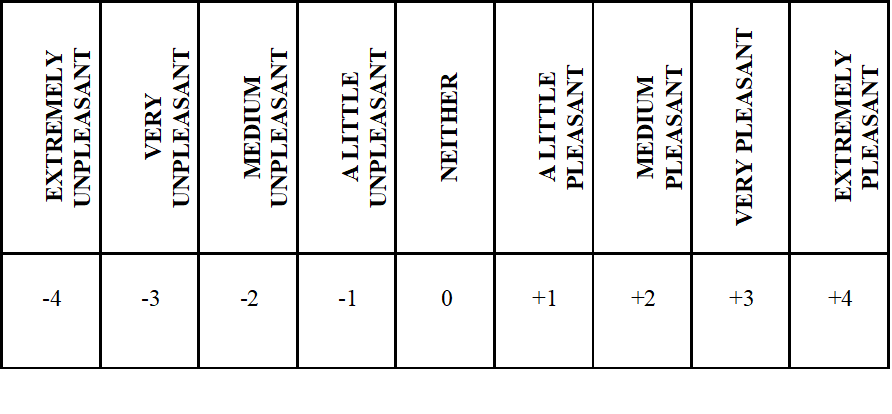
Scheme 3: Bipolar Scale rating
Results of the sensory evaluation (Figures 5 and 6) did not show any extreme reactions of liking in any of the cases. In the case of the infusion samples, Lavender had the least acceptability with 46% of the participants describing the beverage as “Very Unpleasant” or “Extremely Unpleasant”. Similarly, the responses were Medium to Extremely Unpleasant for the Tilia and Sideritis samples at a rate of 36% and 35%, respectively. Finally, Sage samples, did not receive any “Very Unpleasant” or “Extremely Unpleasant” rates, however, 60% of the participants found the beverage moderately acceptable.
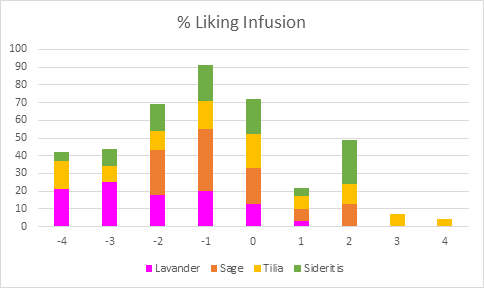
Figure 5: % Liking of Infusion treatment for all beverages (Pivot Chart)
The decoction samples were overall slightly better accepted. In this preparation method, Sage had no negative responses, Lavender’s “Very Unpleasant” or “Extremely Unpleasant “rates were 40%, while the responses of Medium to Extremely Pleasant for the Tilia and Sideritis samples reflected21% and 30% of the participants, respectively.
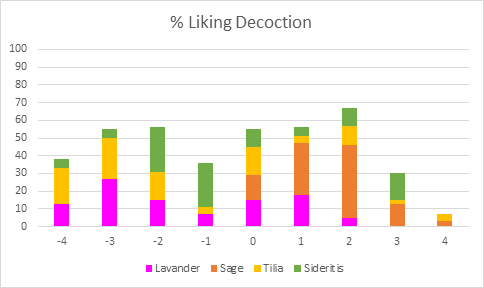
Figure 6: % Liking of Decoction treatment for all beverages (Pivot Chart)
Spices and herbs are rich sources of powerful antioxidants. Spices and herbs have been used for flavour, colour and aroma for more than 2000 years.[107] The Mediterranean diet is currently attracting interest because of its health benefits that may be due, in part, to the high content of this diet in antioxidant phytochemicals.[108]
Our study examined the antioxidant characteristics of four aromatic plants participating in the Mediterranean Diet as beverages. Results showed a strong relation between the antioxidant activity and the phenolic content of each beverage, regardless of the manner of preparation, as the total antioxidant activity, is increased with the content of phenols in most cases. This finding has been previously observed by a number of studies, involving different set of herbs and indicating the role of phytochemicals in this biological activity.[109] Different treatment of the samples appeared to minimally influence the concentration of phenolic compounds, as 3 out of 4 beverages presented higher phenolic content. Sage and Tilia demonstrated the highest rates of antioxidant activity in one or both test (ABTS and/or DPPH), which in the case of Sage can be related to its consisting of flavone glycosides and a range of rosmarinic acid derivatives, as it has been already presented in previous studies.[110]
In all cases, decoction samples presented higher antioxidant results e.g. in the Sage beverage and in the Tilia beverage. Although these two herbs had the highest antioxidant activities, differences between Tilia and Sage beverages were not statistically significant, as it has been previously observed by a previous study.[56] However, further studies showed that Sage’s beverage presented stronger inhibitory activity against linoleic acid peroxidation.
Tilia samples and specifically, those of the infusion treatment were found to be stronger scavengers for hydroxyl radicals compared to the samples of Sideritis, although a recent study indicated that linden flowers beverage extract could not be as important as diet-derived antioxidants in preventing oxidative damage in the tissues by reducing the lipid oxidation or inhibiting the production of ethanol-induced free radicals in rats.[111]
The decoction samples of Lavender beverage presented medium results in relation to the phenolic content, which can be related to the fact that only the flowers of the plant were used, while literature supports that leafy stalks may present higher activity. [112] On the other hand, Sideritis showed better results in the infused samples regarding the phenolic content and the reaction with ABTS and DPPH radical, although at the decoction samples’ scavenging ability was reported stronger.
In both cases (infusion and decoction), there has been intense and steep discoloration in the interaction with DPPH as well as in the interaction with the ABTS cationic radical. This observation was more significant in the case of the more condensed samples, and further experimental study of the action is required.
On the organoleptic evaluation all beverages received moderate reviews. with the main comment from the testers being that the beverage was missing a sweetener and that in lack of it, it would be unlike to be consumed. Finally, beverages prepared as decoctions had slightly better reviews in reference to acceptability, while Sage and Tilia presented a better organoleptic profile.
This study presented a series of Mediterranean herbs that have been used for the preparation of beverages at house conditions. Although all tested beverages exhibited significant antioxidant activity, Sage was found to present the highest antioxidant activity in parallel to high sensory evaluation. Especially, Sage’s beverage, prepared using decoction procedure seems to be the proposed beverage for a preparation at house conditions. There is a wide range of aspects that need to be tested in the future in order to provide a better understanding of the necessity of including aromatic plants’ beverages in regular diet.
The authors would like to thank Anainta Ananian, Maria Charitonos, Barbara Kasimidou, Anastasia Kimoglou, Marianna Mpakali, Andromachi Mpala, Chrisanthi Rousaki and Eirini Vasiari for their contribution to the preliminary parts of this study.
Funding. This research received no specific grant from any funding agency in the public, commercial or not-for profit sectors.
Petrovska B. B., 2012, Historical review of medicinal plants' usage, Pharmacogn. Rev., 6: 1–5. PMid:22654398 PMCid:PMC3358962
View Article PubMed/NCBICasta-er O, Fitó M, López-Sabater MC, Poulsen HE, Nyyssönen K, Schröder H, Salonen JT, De la Torre-Carbot K, Zunft HF, De la Torre R, Bäumler H, Gaddi AV, Saez GT, Tomás M, Covas MI, 2011, The effect of olive oil polyphenols on antibodies against oxidized LDL. A randomized clinical trial, Clin. Nutr., 30, 490–493. PMid:21376434
View Article PubMed/NCBIKnoops. KB, de G. Lisette M, D. Kromhout, and et al, 2004, Mediterranean diet, lifestyle factors, and 10-year mortality in elderly european men and women: The hale project, JAMA, 292, 1433–1439.
Esposito K. et al., 2004 Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial., JAMA, 292, 1440-1446. PMid:15383514
View Article PubMed/NCBIBenjamin E. J. et al., 2017 Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association, Circulation, 2017.
View ArticleCarlini E. A., 2003, Plants and the central nervous system," Pharmacology Biochemistry and Behavior. 00112-6
View ArticleRodriguez-Mateos Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A., 2014 Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update, Archives of Toxicology. 88, 1803-55 PMid:25182418
View Article PubMed/NCBIShahidi F., 2012, Nutraceuticals, functional foods and dietary supplements in health and disease," in Journal of Food and Drug Analysis, 20, 226-230.
Trichopoulou A., Bamia C., and Trichopoulos D., 2009 "Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study," BMJ: 338
View ArticlePrasad C. * Imrhan V, Juma S, Maziarz M, Prasad A, Tiernan C and Vijayagopal P, Bioactive plant metabolites in the management of non-communicable metabolic diseases: Looking at opportunities beyond the horizon, Metabolites. 5: 733-765 PMid:26703752 PMCid:PMC4693193
View Article PubMed/NCBIde Pascual-Teresa S., Moreno D. A., and García-Viguera C., 2010 Flavanols and anthocyanins in cardiovascular health: A review of current evidence, International Journal of Molecular Sciences. 13:1679-1703. PMid:20480037 PMCid:PMC2871133
View Article PubMed/NCBIMukhtar M., Arshad M., Ahmad M., Pomerantz R. J., Wigdahl B. and Parveen Z., 2008 Antiviral potentials of medicinal plants, Virus Research. 131: 111-120. PMid:17981353
View Article PubMed/NCBIGurib-Fakim A., 2006 Medicinal plants: Traditions of yesterday and drugs of tomorrow, Molecular Aspects of Medicine. 27:1-93. PMid:16105678
View Article PubMed/NCBIVladimir-kneževi S. and Blažekovi B., "Plant Polyphenols as Antioxidants Influencing the Human Health," Phytochem. as Nutraceuticals – Glob. Approaches to Their Role Nutr. Heal., 2012.
View ArticleRice-Evans C., Miller N., and Paganga G., 1997 Antioxidant properties of phenolic compounds, Trends Plant Sci., 2: 152-159 01018-2
View ArticleCarlsen M. H., Halvorson BL et al., 2010 The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide, Nutr. J., 9: 3 PMid:20096093 PMCid:PMC2841576
View Article PubMed/NCBIMiddleton E., Kandaswami C., and Theoharides T. C., 2000, The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer., Pharmacol. Rev., 52: 673-751. PMid:11121513
PubMed/NCBIKumar S. and Pandey A. K., 2013, Chemistry and biological activities of flavonoids: An overview, The Scientific World Journal. PMid:24470791 PMCid:PMC3891543
View Article PubMed/NCBIWilliams R. J., Spencer J. P. E., andRice-Evans C., 2004, Flavonoids: Antioxidants or signalling molecules?, Free Radical Biology and Medicine.36: 838-849. PMid:15019969
View Article PubMed/NCBIPanche A. N., Diwan A. D., and Chandra S. R., 2016 Flavonoids: An overview, Journal of Nutritional Science. 5:e47 PMid:28620474 PMCid:PMC5465813
View Article PubMed/NCBIDudonné, S. Vitrac X., Coutiére P., Woillez M., and Mérillon J. M., 2009, Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays, J. Agric. Food Chem., 57: 1768-1774. PMid:19199445
View Article PubMed/NCBIMcCullough M. L., Peterson J. J., Patel R., Jacques P. F., Shah R., and Dwyer J. T., 2012, Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults, Am. J. Clin. Nutr., 95: 454–464. PMid:22218162 PMCid:PMC3260072
View Article PubMed/NCBIPeterson J. J., Dwyer J. T., Jacques P. F., and McCullough M. L., 2012, Do Flavonoids Reduce Cardiovascular Disease Incidence or Mortality in US and European Populations?, Nutr. Rev., 70:491–508. PMid:22946850 PMCid:PMC4130174
View Article PubMed/NCBIDemo A., Petrakis C., Kefalas P., and Boskou D., 1998, Nutrient antioxidants in some herbs and Mediterranean plant leaves," Food Res. Int., 31:351-354 00086-6
View ArticleEstruch R. et al., 2006 Effects of a Mediterranean-Style Diet on Cardiovascular Risk FactorsA Randomized Trial," Ann. Intern. Med., 145:1-11. PMid:16818923
View Article PubMed/NCBIBabio N., Bulló M., andSalas-Salvadó J., 2009, Mediterranean diet and metabolic syndrome: The evidence, Public Health Nutr., 121607-1717
View ArticleMartínez-González M. A., Salas-Salvadó J., Estruch R., Corella D., Fitó M., and Ros E., 2015, Benefits of the Mediterranean Diet: Insights From the PREDIMED Study, Prog. Cardiovasc. Dis., 58: 50-60. PMid:25940230
View Article PubMed/NCBIB. Burlingame and S. Dernini, "Sustainable diets: the Mediterranean diet as an example.," Public Health Nutr., 2011. PMid:22166185
View Article PubMed/NCBIF. Algieri et al., "Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L.," J. Ethnopharmacol., 2016.
View ArticleMishawaka Charles Denys J., Antioxidant properties of spices, herbs and other sources. Springer, 2012.
Hofrichter J. et al., 2016 , Sideritis spp. Extracts Enhance Memory and Learning in Alzheimer's β-Amyloidosis Mouse Models and Aged C57Bl/6 Mice," J. Alzheimer's Dis., 53967-980
View ArticleKiritsakis K., Kontominas M. G., Kontogiorgis C., Hadjipavlou-Litina D., Moustakas A. and Kiritsakis 2010, Composition and antioxidant activity of olive leaf extracts from Greek olive cultivars," JAOCS, J. Am. Oil Chem. Soc.,.87: 369-376
Daferera D. J., Ziogas B. N., and Polissiou M. G., 2000 GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum, J. Agric. Food Chem., 48:2576-2581. PMid:10888587
View Article PubMed/NCBISkotti E., Anastasaki E., Kanellou G., Polissiou M., and Tarantilis P. A., 2014, Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants, Ind. Crops Prod., 53: 46-54.
View ArticleProestos C. and Komaitis M., 2013, Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation, Foods, 2:90-99. PMid:28239100 PMCid:PMC5302235
View Article PubMed/NCBIPombal S. et al., 2016, Antibacterial and antioxidant activity of Portuguese Lavandula luisieri (Rozeira) Rivas-Martinez and its relation with their chemical composition," Springerplus, 5:171. PMid:27777848 PMCid:PMC5050179
View Article PubMed/NCBINikolova G, Karamalakova Y, Kovacheva N, Stanev S, Zheleva A, Gadjeva V. Protective effect of two essential oils isolated from Rosa damascena Mill. and Lavandula angustifolia Mill, and two classic antioxidants against L-dopa oxidative toxicity induced in healthy mice. - Regul Toxicol Pharmacol. 2016 Nov;81:1-7. PMid:27381452
View Article PubMed/NCBIGülçin Ì., Şat I. G., Beydemir Ş., Elmastaş M., and Küfrevioǧlu Ö. I., 2004, Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.), Food Chem., 87: 393-400.
View ArticleNatural Medicines, "Natural Medicines - Professional," Therapeutic Research Center, 2015.
Koulivand P. H., Khaleghi Ghadiri M., and Gorji A., 2013, Lavender and the nervous system, Evidence-based Complement. Altern. Med., 681304.
Vakilian K., Atarha M., Bekhradi R., and Chaman R, 2011., Healing advantages of lavender essential oil during episiotomy recovery: A clinical trial, Complement. Ther. Clin. Pract., 17:50-53 PMid:21168115
View Article PubMed/NCBIRaisi Dehkordi Z., Hosseini Baharanchi F. S., and Bekhradi R., 2014, Effect of lavender inhalation on the symptoms of primary dysmenorrhea and the amount of menstrual bleeding: A randomized clinical trial, Complement. Ther. Med., 22:212-219. PMid:24731891
View Article PubMed/NCBISheikhan F., Jahdi F., Khoei E. M., Shamsalizadeh N., Sheikhan M., and Haghani H., 2012, Episiotomy pain relief: Use of lavender oil essence in primiparous Iranian women," Complement. Ther. Clin. Pract.18:66-70. PMid:22196577
View Article PubMed/NCBISasannejad P., Saeedi M., Shoeibi A., Gorji A., Abbasi M., and Foroughipour M., 2012, Lavender essential oil in the treatment of migraine headache: A placebo-controlled clinical trial, Eur. Neurol., 67:288-291. PMid:22517298
View Article PubMed/NCBICavanagh H. M. A. and Wilkinson J. M., 2002, Biological activities of lavender essential oil, Phytotherapy Research. 16:301-308. PMid:12112282
View Article PubMed/NCBICavanagh H. M. A. and Wilkinson J. M., 2005, Lavender essential oil: a review," Aust. Infect. Control, 10:35-37
View ArticleFismer K. L. and Pilkington K., 2012, Lavender and sleep: A systematic review of the evidence, European Journal of Integrative Medicine. 4:e436-e447
View ArticleHernández-Pérez M. and Rabanal R. M., 2002, Evaluation of the antinflammatory and analgesic activity of Sideritis canariensis var. pannosa in mice," J. Ethnopharmacol., 81:43-47. 00033-8
View ArticleKassi E. et al., 2013 Sideritis euboea extract lowers total cholesterol but not LDL cholesterol in humans: A randomized controlled trial, Clinical Lipidology. 8:627-634
View ArticleBojovic D., Jankovic S., Potpara Z., and Tadic V., 2011 Summary of the phytochemical research performed to date on sideritis species, Serbian J. Exp. Clin. Res. 12: 898-905.
View ArticleWalbroel B. and Feistel B., 2010, Greek Mountain Tea – an herbal drug for mental enhancement, in 58th International Congress and Annual Meeting of the Society for Medicinal Plant and Natural Product Research,.76
View ArticleKorou L.-M. et al., 2016, Medicinal properties of Mediterranean plants against glucose and lipid disorders, J. Med. Plants Stud. JMPS, 4: 94-100.
Stansbury J., Saunders P., and Winston D., 2012, Supporting Adrenal Function with Adaptogenic Herbs," J. Restor. Med., 1:76-82.
View ArticleDemiray S., Pintado M., and Castro P., 2009, Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots, World Acad. Sci. Eng. Technol., 3:74-79.
Loppi S. and Frati L., "Influence of Tree Substrate on the Diversity of Epiphytic Lichens: Comparison Between Tilia platyphyllos and Quercus ilex (Central Italy)," Bryologist, 107:340-344. 107[0340:IOTSOT]2.0.CO;2
View ArticleYildirim A., Mavi A., Oktay M., Kara A. A., Algur O. F., and Bilaloglu V., 2000, Comparison of antioxidant and antimicrobial activities of Tilia (Tilia argentea Desf ex DC), sage (Salvia triloba L.), and Black tea (Camellia sinensis) extracts," J. Agric. Food Chem., 48:5030-5034. PMid:11052773
View Article PubMed/NCBIBommer S., Klein P., and Suter A., 2011, First time proof of sage's tolerability and efficacy in menopausal women with hot flushes, Adv. Ther., 28:490-500. PMid:21630133
View Article PubMed/NCBIGhorbani A. and Esmaeilizadeh M., 2017, Pharmacological properties of Salvia officinalis and its components," J. Tradit. Complement. Med., 7:433-440. 59. Perry N. S. L., Bollen C., Perry E. K., and Ballard C., 2003, Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial," Pharmacology Biochemistry and Behavior. 75:651-659.
Perry N. S. L., Bollen C., Perry E. K., and Ballard C., 2003, Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial,” Pharmacology Biochemistry and Behavior. 75:651-659.
da Silva G. L. et al., 2015, Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil, An. Acad. Bras. Cienc., 87:1397-1408. PMid:26247152
View Article PubMed/NCBIPanuccio M. R., Fazio A., Papalia T., and Barreca D., 2016, Antioxidant Properties and Flavonoid Profile in Leaves of Calabrian Lavandula multifida L., an Autochthon Plant of Mediterranean Southern Regions, Chem. Biodivers., 13:416-421. PMid:26948403
View Article PubMed/NCBINzeako B. C., Al-Kharousi Z. S. N., and Al-Mahrooqui Z., 2006, Antimicrobial activities of clove and thyme extracts, Sultan Qaboos Univ. Med. J., 6:33-39. PMid:21748125 PMCid:PMC3074903
PubMed/NCBIMata A. T., Proença C., Ferreira A. R., Serralheiro M. L. M., Nogueira J. M. F., and Araújo M. E. M., "Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices," Food Chem., 103:778-786.
View ArticleRoby M. H. H., Sarhan M. A., Selim K. A. H., and Khalel K. I., 2013, Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts, Ind. Crops Prod., 72:210-214.
View ArticleYashin A., Yashin Y., Xia X., and Nemzer B., 2017, Antioxidant Activity of Spices and Their Impact on Human Health: A Review," Antioxidants, 6:E70. PMid:28914764 PMCid:PMC5618098
View Article PubMed/NCBIMartin K. R., 2009, Polyphenols as dietary supplements: A double-edged sword, Nutr. Diet. Suppl., 2:1-12.
View ArticleLo Y. T., Li M., and Shaw P. C., 2015, Identification of constituent herbs in ginseng decoctions by DNA markers, Chinese Med. (United Kingdom), 10"1-8.
View ArticleTing A., Chow Y., and Tan W., 2013, Microbial and heavy metal contamination in commonly consumed traditional Chinese herbal medicines, J. Tradit. Chinese Med., 33:119-124 60112-0
View ArticleYang R. Y., Tsou S. C. S., Lee T. C., Chang L. C., Kuo G., and Lai P. Y., 2006 , Moringa, a novel plant rich in antioxidants, bioavailable iron, and nutrients, Herbs Challenges Chem. Biol.,.224-239
Proestos C, Boziaris I.S, Kapsokefalou M., Komaitis M. 2008, Natural antioxidant constituents from selected aromatic plants and their antimicrobial activity against selected pathogenic microorganisms. Food Technology and Biotechnology. 46: 151-156.
Robbins Rebecca J. 2003, Phenolic Acids in Foods: An Overview of Analytical Methodology. Journal of Agricultural and Food Chemistry. 51: 2866–2887 PMid:12720366
View Article PubMed/NCBIGrassmann, J. et Elstner, E. F. 2003, Essential oils. Properties and uses. Encyclopedia of Food Sciences and Nutrition. Academic Press, 2177-2184. PMCid:PMC275419
Kontogiorgis C., Deligiannidou G-E., Hadjipavlou-Litina D., Lazari D., Papadopoulos A., 2016, Antioxidant protection: The contribution of proper preparation of fennel (Foeniculum vulgare Mill.) beverage. Industrial Crops and Products, 79: 57-62.
View ArticleCui D-N.,Wang X.,Chen J-Q., B. Lv, Zhang P.,Zhang W.,Zhang Z-J.,Xu F-G. 2017, Quantitative Evaluation of the Compatibility Effects of Huangqin Decoction on the Treatment of Irinotecan-Induced Gastrointestinal Toxicity Using Untargeted Metabolomics. Front Pharmacol. 8: 211 PMid:28484391 PMCid:PMC5399027
View Article PubMed/NCBIGraf Ernst. 1992, Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13:435-48. 90184-I
View ArticleRe R., Pellegrini N., Proteggente A., Pannala A., Yang M., and Rice-Evans C., "Antioxidant activity applying an improved ABTS radical cation decolorization assay," Free Radic. Biol. Med., 26:1231-1237. 00315-3
View ArticleArts M. J. T. J., Haenen G. R. M. M., Voss H. P., and Bast A., 2004, Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay, Food Chem. Toxicol., 42:45-49 PMid:14630129
View Article PubMed/NCBIMiller N. J. and Rice-Evans C. A., 1997, Factors Influencing the Antioxidant Activity Determined by the ABTS •+ Radical Cation Assay," Free Radic. Res. 26:195-199 PMid:9161842
View Article PubMed/NCBIOh J., Jo H., Cho A. R., Kim S. J., and Han J., 2013 Antioxidant and antimicrobial activities of various leafy herbal teas," Food Control, 31:403-409
View ArticleDorman H. J. D., Peltoketo A., Hiltunen R., and Tikkanen M. J., 2003, Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs, Food Chem., 83:255-262. 00088-8
View ArticleFloegel A., Kim D. O., Chung S. J., Koo S. I., and Chun O. K., 2011, Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods," J. Food Compos. Anal., 20:169-175.
View ArticleBrand-Williams W., Cuvelier M. E., and Berset C., 1995, Use of a free radical method to evaluate antioxidant activity," LWT - Food Sci. Technol., 28:25-30
Kulišić T., Dragović-Uzelac V., and Miloš M., 2006, Antioxidant activity of aqueous tea infusions prepared from oregano, thyme and wild thyme," Food Technol. Biotechnol., 44:485-492
Kontogiorgis C., Deligiannidou G-E., Hadjipavlou-Litina D., Lazari D., Papadopoulos A., 2016Antioxidant protection: The contribution of proper preparation of fennel (Foeniculum vulgare Mill.) beverage. Industrial Crops and Products, 79, 57-62.
View ArticleKontogiorgis C. and Hadjipavlou-Litina D., 2005, Synthesis and anti-inflammatory activity of coumarin derivatives. J Med Chem; 48:6400-6408 PMid:16190766
View Article PubMed/NCBIPontiki E. and Hadjipavlou-Litina D., 2007, Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents, Bioorganic Med. Chem., 15:5819-5827 PMid:17604175
View Article PubMed/NCBISimplicio F. I., Seabra A. B., De Souza G. F. P., and De Oliveira M. G., "In vitro inhibition of linoleic acid peroxidation by primary S-Nitrosothiols," J. Braz. Chem. Soc., 21:1787-1806.
View ArticleRajic Z, Hadjipavlou-Litina D, Pontiki E, Kralj M, Suman L, Zorc B. 2010, The novel ketoprofen amides–synthesis and biological evaluation as antioxidants, lipoxygenase inhibitors and cytostatic agents. Chem Biol Drug De,75:641–652 PMid:20337784
View Article PubMed/NCBIDominy N. J., Lucas P. W., Osorio D., andYamashita N., 2001, The sensory ecology of primate food perception, Evolutionary Anthropology. 10:171-186.
View ArticleAdnan M., Ahmad A., Ahmed A., Khalid N., Hayat I., and Ahmed I., "Chemical Composition and Sensory Evaluation of Tea (Camellia Sinensis) Commercialized in Pakistan," Pak. J. Bot, 45: 901-907.
Havermans R. C., Janssen T., Giesen J. C. A. H., Roefs A., and Jansen A., 2009, Food liking, food wanting, and sensory-specific satiety, Appetite, 52:222-225. PMid:18951934
View Article PubMed/NCBILawless H. T. and Heymann H., 2010Sensory Evaluation of Food. 1-19
Drewnowski, A. 1997, Taste Prederences and Food Intake, Annu. Rev. Nutr., 17:237-253. PMid:9240927
View Article PubMed/NCBISingh-Ackbarali D. and Maharaj R., 2014, Sensory Evaluation as a Tool in Determining Acceptability of Innovative Products Developed by Undergraduate Students in Food Science and Technology at The University of Trinidad and Tobago," J. Curric. Teach., 3:10-27
View ArticlePeinado I., Girón J., Koutsidis G., and Ames J. M., 2014, Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds, Food Res. Int., 66:36-44.
View ArticleBourn D. and Prescott J., 2002 A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods," Crit. Rev. Food Sci. Nutr., 42, 1-34 PMid:11833635
View Article PubMed/NCBIAinsworth E. A. and Gillespie K. M., 2007, Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent," Nat. Protoc., 2:875-877. PMid:17446889
View Article PubMed/NCBIScaduto RC Jr. 1995, Oxidation of DMSO and methanesulfinic acid by the hydroxyl radical. Free Radic Biol Med. 18:271-7. E0139-A
View ArticleKeskes H, Belhadj S, Jlail L, El Feki A, Damak M, Sayadi S, Allouche N, 2017 LC-MS–MS and GC-MS analyses of biologically active extracts and fractions from Tunisian Juniperus phoenice leaves. Pharmaceutical Biology, 55:88-95. PMid:27925471
View Article PubMed/NCBISacchetti G. et al., 2004, Composition and functional properties of the essential oil of Amazonian basil, Ocimum micranthum Willd., Labiatae in comparison with commercial essential oils," J. Agric. Food Chem., 52,3486-3491. PMid:15161220
View Article PubMed/NCBIHu C., Yuan Y. V., and Kitts D. D., 2007, Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro, Food Chem. Toxicol., 45:2219-2227. PMid:17624649
View Article PubMed/NCBILesser M. P., 2006, Oxidateive Stress in Marine Environments: Biochemistry and Physiological Ecology," Annu. Rev. Physiol., 68:253-278. 42. Gligorovski S., Strekowski R., Barbati S., and Vione D., 2015, Environmental Implications of Hydroxyl Radicals (•OH)," Chem. Rev., 115, 13051-13092.
Gligorovski S., Strekowski R., Barbati S., and Vione D., 2015, Environmental Implications of Hydroxyl Radicals (•OH),” Chem. Rev., 115, 13051-13092.
Kontogiorgis C. A., Bompou E.-M., Ntella M., and Vanden Berghe W., 2010, Natural Products from Mediterranean Diet: From Anti-Inflammatory Agents to Dietary Epigenetic Modulators, Antiinflamm. Antiallergy. Agents Med. Chem., 9:
View ArticlePannangpetch P., Laupattarakasem P., Kukongviriyapan V., Kukongviriyapan U., Kongyingyoes B., and Aromdee C., 2007, Antioxidant activity and protective effect against oxidative hemolysis of Clinacanthus nutans (Burm . f) Lindau, Songklanakarin J. Sci. Technol., 29:Suppl 1.
Somparn P., Phisalaphong C., Nakornchai S., Unchern S., and Morales N. P., 2007, Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives., Biol. Pharm. Bull., 30:74-78 PMid:17202663
View Article PubMed/NCBIEmbuscado M. E., 2015, Spices and herbs: Natural sources of antioxidants - A mini review," Journal of Functional Foods. 18:811-819
View ArticleVasilopoulou E., Georga K., Joergensen M., Naska A., and Trichopoulou A., 2005, The Antioxidant Properties of Greek Foods and the Flavonoid Content of the Mediterranean Menu, Curr. Med. Chem. Endocr. Metab. Agents, 5:33-45.
View ArticleMilan S., 2011, Total Phenolic Content, Flavonoid Concentration and Antioxidant Actibity of Marrubium peregrinum L. Extracts, Kragujev. J. Sci, 33, 63.72.
Lu Y. and Yeap Foo, 2001, Antioxidant activities of polyphenols from sage (Salvia officinalis), Food Chem., 75:197-202 00198-4
View ArticleYayalaci Y., Celik I., and Bati B., 2014, Hepatoprotective and antioxidant activity of linden (Tilia platyphyllos L.) infusion against ethanol-induced oxidative stress in rats, J. Membr. Biol., 247:181-188. PMid:24337514
View Article PubMed/NCBIAdaszyńska-Skwirzyńska M. and Dzięcioł M., 2017, Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland," Nat. Prod. Res., 31:2575-2580. PMid:28449600
View Article PubMed/NCBI