Chin-Chu Chen
ADDRESS: Chin-Chu Chen, PhD. No. 60, Sec. 3, Long-gang Rd., Zhong-Li City, Taoyuan County 320, Taiwan.
Tel.:+886 3 4572121; fax: +886 3 4572128.
E-mail: gkbioeng@grapeking.com.tw
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 2 ISSUE: 1
Page No: 150-156
Chin-Chu Chen
ADDRESS: Chin-Chu Chen, PhD. No. 60, Sec. 3, Long-gang Rd., Zhong-Li City, Taoyuan County 320, Taiwan.
Tel.:+886 3 4572121; fax: +886 3 4572128.
E-mail: gkbioeng@grapeking.com.tw
Li ICa, Hsu JHa, Lin TWa, Lin WHb and Chen CCa,c,d*
aGrape King Bio Ltd, Zhong-Li Dist., Taoyuan City, Taiwan
bSchool of Pharmacy, China Medical University, Taichung, Taiwan.
cInstitute of Food Science and Technology, National Taiwan University, Taipei, Taiwan.
dDepartment of Food Science, Nutrition and Nutraceutical Biotechnology, Shih Chien University, Taipei City, Taiwan
Dr. Adalisa Ponzano(adalisa@hotmail.it)
Dr. Alex Boye(boye_alex@yahoo.com)
Prof.Dr. Refiye Yanardag(refiyeyanardag@yahoo.com)
I-Chen Li, Hsu JH; Lin TW; Lin WH and Chen CC, Prenatal Developmental Toxicity Study of HEA-enriched Cordyceps Cicadae Mycelia in Sprague-Dawley Rats(2017)SDRP Journal of Food Science & Technology 2(1)
Background:
Cordyceps cicadae (C. cicadae) has been used in traditional medicine and is presently being developed for therapeutic purposes and dietary supplements. One of its effective bioactive ingredients is N6-(2-hydroxyethyl) adenosine (HEA), which can exert an analgesic effect by inhibiting neurotransmitter release and can be used to ameliorate moderate to severe pain, including cancer pain. Although there have been extensive studies confirming its safety, the embryo-fetal development toxicity of HEA-enriched C. cicadae mycelia has not been evaluated.
Methods:
This study was to describe the potential adverse effects of orally administered HEA-enriched C. cicadae mycelia on fertility and early embryonic development to implantation in Sprague-Dawley rats at doses of 500, 1,000, and 1,500 mg/kg.
Results:
No external, visceral as well as skeletal abnormalities were noted among all groups. There were no statistically significant differences in the number of fetuses, corpora lutea, implantation sites, embryo resorptions, stillbirths, and post-implantation between all dosing groups. Moreover, there was no embryo-fetal developmental toxicity observed.
Conclusion:
The no-observed-adverse-effect level (NOAEL) of HEA-enriched C. cicadae mycelia was considered to be greater than 1500 mg/kg. As there was no evidence of teratogenic effect, HEA-enriched C. cicadae mycelia were therefore not considered as a potent embryotoxin in rats.
Keywords:
N6-(2-hydroxyethyl) adenosine; traditional medicine; teratogenicity; no-observed-adverse-effect level.
Cordyceps cicadae (C. cicadae), also named as “Chan Hua” in Chinese, is an entomopathogenic and parasitic fungus that belongs to the family Clavicipitaceae, Ascomycotina[1]. The mycelia of this fungus grow inside the nymph of specific hosts (Cicada flammata Distant, Platypleura kaempferi Fabricius, Crytotympana pustulata Fabricious, Platylomia pieli Kato and Oncotympana maculatieollis Motsch) and then forms fruiting bodies on the surface of these insects[2]. C. cicadae has been used as both food and Traditional Chinese Medicine since 1580 years ago, which was much longer than the use of Ophicordyceps sinensis[3], one of the most famous traditional Chinese medicines and medicinal mushrooms. In the long history of medicine Cordyceps cicadae has been used, it has been primarily used in the treatment of childhood palpitation, epilepsy, convulsions, and many eye diseases[4]. Recently, extensive pharmacological properties of C. cicadae were reported, including renoprotective attributes[5], anti-hypoglycemic properties, anti-tumor properties[6], analgesic activity, sedative function[7], anti-fatigue effects[8] and immunomodulatory effects[9].
N6-(2-hydroxyethyl) adenosine (HEA), an adenosine derivative, is one of the main bioactive ingredients from C. cicadae. Since 1980s, researchers have confirmed that endogenous nucleosides and their analogues may be involved in pain modulation[10, 11]. HEA can bind to α1 receptors, thereby exerting an analgesic effect by inhibiting neurotransmitter release[12]. Moreover, its chemical structure is different from that of opioid analgesics so there is no dependence developed following the use of HEA for pain relief, which can be safely used for clinical application[13]. Recently, a well-designed, preclinical study has been conducted to demonstrate that HEA-containing C. cicadae fermentation product has 99% acetic acid-induced writhing-reducing effect in Kunming mice, which was higher than that of morphine[14]. Hence, there is potential in developing C. cicadae enriched with HEA as an ingredient in functional foods or medicinal products intended to ameliorate moderate to severe pain, including cancer pain.
At present, production of HEA by various Cordyceps species has been one of the most extensively explored areas in recent years owing to its enormous commercial value[15]. While HEA-enriched C. cicadae seems promising, its safety concern has not been evaluated and should be addressed. Myriocin (ISP-1/thermozymocidin), for example, is a common secondary metabolite purified from cultured filtrates of Isaria sinclairii parasite on the larva of the cicada but is known to induce severe disorders, resulting in the death of the animals at a dose of 1 mg/ml[17]. Although an extensive set of toxicology studies on C. cicadae has been investigated[18-20], the teratogenic effects of this insect-fungus complex on the embryo during pregnancy have not been evaluated. This is the first study aims to examine the influence of HEA-enriched C. cicadae mycelia broth on embryo-fetal development in Sprague-Dawley rats to certify its safety as a functional food ingredient.
Sample preparation
Proximate, myriocin and HEA analyses
Detailed descriptions of the proximate and HEA analyses were presented in the previous study[20]. Myriocin analysis was performed on a liquid chromatography tandem mass chromatography (LC-MS/MS) system. An Agilent 1100 series LC system (Agilent, Waldbronn, Germany) equipped with a G1376A capillary pump and a G1313A autosampler was applied. Chromatographic separation was achieved on a Luna C18 column (5 µm, 150×2.0 mm, Phenomenex Inc., Madrid Avenue Torrance, CA, USA) at 25 ⁰C. The mobile phase consisted of water (A) and acetonitrile (B), and the gradient was as follows: 0 min: 40 % B, 0-10 min: 40-100 % B, 10-15 min: 100 % B, 15-16 min: 100-40 % B, and 16-20 min: 40 % B, with a flow rate of 0.3 mL/min. Mass spectrometric detection was performed on an API 3000 triple quadrupole instrument (Applied Biosystems, Concord, Ontario, Canada) in multiple reaction monitoring (MRM) mode. A turbo ionspray electrospray ionization (ESI) interface in positive ionization mode was operated using the following settings: ion spray voltage = 4,500 V, ion source heater temperature = 300°C, Nebulizer Gas = 8 psi, Collision Gas = 7 psi, and curtain gas setting = 7 psi. Data processing was performed with Analyst 1.4.2 software (Applied Biosystems, Concord, Ontario, Canada).
Animals and Husbandry
110 pregnant Sprague-Dawley rats at 8 weeks of age (BioLASCO Taiwan Co., Ltd., Ilan, Taiwan) were acclimatized for three days prior to the initiation of the study. The animals were housed in autoclaved polyethylene cages (one per cage), kept in standard conditions of temperature (22 ± 3°C) and illumination cycle (12:12 h light and dark schedule), fed with MFG diet (Oriental Yeast Co., Ltd, Tokyo, Japan), and given ad libitum access to water. This protocol was approved by the Institutional Animal Care and Use Committee (IACUC:101-12i) and carried out in full compliance with Good Laboratory Practice (GLP).
Study design
This study was performed under the guidelines of Organization for Economic Co-operation and Development (OECD) 414 (Prenatal Developmental Toxicity Study)[21]. Using a random number generator (Excel; Microsoft, Redmond, WA), the experimental animals were divided randomly into 5 groups (a minimum of 20 animals per group) as follows: three dosages test groups, one negative control group and one positive group. The negative control group received vehicle treatment (10 ml/kg distilled water daily), the positive control group received 10 ml/kg aspirin (Sigma-Aldrich) at a dosage of 250 mg/kg body weight (bw)[22] while the three dosages test groups received 10 ml/kg HEA-enriched C. cicadae mycelia at a dose of 500 (low dose), 1,000 (mid dose) and 1,500 (high dose) mg/kg bw, respectively. These dose levels were selected according to the previous chronic toxicity study[20] and were administered orally in the gestation of 6 to 15 days, which is defined as the critical period for early fetal structural development. The day a vaginal plug and/or sperm in vaginal smear were observed was defined as day 0 of pregnancy.
Average measurements of body weight and food were conducted daily during the study period. On the 20th day of pregnancy, the rats were anesthetized with pentobarbital, and the fetuses were delivered by caesarean sections. All fetuses were weighed and the corpora lutea, implantation, absorbed fetus, and viable fetus were numbered. Individual viable offsprings was then assessed for observable abnormalities such as anencephaly, cerebral swelling, exencaphaly, cranioschisis, hydrocephaly, microcephaly, microphthalmia, anophthalmia, exophthalmos, anotia, microtia, low-set ears, agnathia, micrognathia, cleft palate, cheilognathopalatoschisis, thoracoschisis, thoracogastroschisis, spina bifida, scoliosis, lordosis, kyphosis, hypospadias, anal atresia, short tail, coiled tail, acaudia, omphalocele, polymelia, dysmelia, ectromelia, hemimelia, polydactyly, adactyly, syndactyly, brachhydactyly, and ectrodactyly. Half of the fetuses in each litter of the negative control group, high dose HEA-enriched C. cicadae treated group and positive control group then underwent bone examinations while the remaining half of the fetuses underwent organ tissue examinations. The visceral examination of fetuses was performed according to previous studies[23, 24]. For skeletal examination, the fetuses were performed after clearing 5% formalin-fixed fetuses with KOH and staining the skeleton with Alizarin Red S. Skeletal development was evaluated according to previous methods[25]. All animals will undergo tissue and bone examinations if abnormalities were found in the high dose group.
Statistical analyses
All data were expressed as means and standard deviations (SD) and analyzed by SPSS statistical software. One-Way ANOVA followed by Duncan test were executed to determine the significant difference between the treatment and control groups. The significance level was set as 5%.
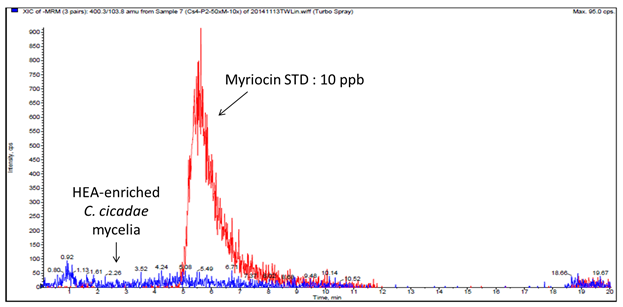
The proximate and HEA analyses have been described in our previous study[20]. Besides HEA, myriocin production is also known to occur in C. cicadae mycelia[28]. Although myriocin has attracted a great deal of attention due to its potent immunosuppressive activity, treatment with myriocin can exert severe gastrointestinal side effects[17]. Since there is an opportunity to develop HEA-enriched C. cicadae mycelia as a functional food ingredient, the presence of myriocin in HEA-enriched C. cicadae mycelia was investigated by using a LC-MS/MS method as a follow-up to the previous study (Figure 1). In this study, HEA-enriched C. cicadae mycelia was cultivated under 25 °C whereas the optimum temperature for myriocin production was found to be 43°C, suggesting the absence of myriocin contents from the same parent compounds. Based on these results, the safe consumption of HEA-enriched C. cicadae mycelia supports its commercial potential and health benefits.
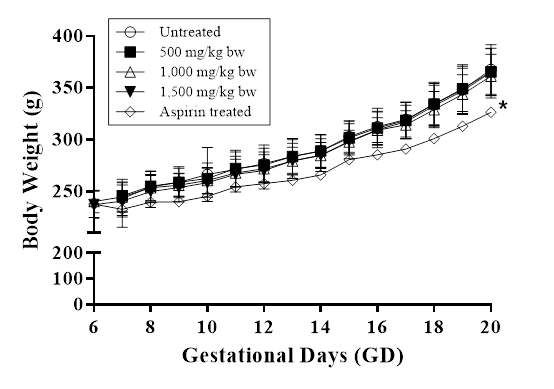
A major concern in the development of functional foods is the accurate assessment of safety for human consumption. Prior to this study, toxicological investigations on HEA-enriched C. cicadae mycelia for a period of 90 days in animals did not show any toxicity up to 2 g/kg oral dose[20]. However, further research is needed to ascertain the effects of HEA-enriched C. cicadae mycelia on prenatal development. In the present study, treatment with HEA-enriched C. cicadae mycelia during the gestation period showed no signs of clinical abnormalities, miscarriages or deaths in any of the pregnant rats. The average weights of the treated female rats in various groups are shown in Figure 2. All treatment groups exposed to HEA-enriched C. cicadae mycelia did not differ from the negative control group in terms of body weight gain and level of food and water consumption (data not shown). In contrast, treatment with aspirin (positive control) profoundly showed weight loss in the pregnant rats, accounting for the differences in body weight when compared to the negative control group on the 20th day (p<0.05), which were in accordance with findings from a previous study[22].
Moreover, the success of reproduction was not significantly affected in the HEA-enriched C. cicadae mycelia treatment groups by measuring different gestational parameters (Table 1), indicating that HEA-enriched C. cicadae mycelia had no adverse effect on maturational and developmental milestones in offsprings. The number of fetuses, corpora lutea, implantation sites, embryo resorptions, stillbirths, and post-implantation loss was similar among the experimental groups (p>0.05). Concerning the fetal variables, the mean birth weight of the experimental pups was 3.64±0.87 for the negative control group and 3.85±0.91, 3.69±0.86 and 3.66±0.84 g for the low, mid and high HEA-enriched C. cicadae mycelia treated groups, respectively. Although there was a statistically significant increase in fetal weight in the low dose group (p<0.05), there was no dose-response. Besides this aspect, there were no distinguishable differences among the sex ratio of the fetuses in all groups (p>0.05). On the other hand, when dams were treated with aspirin, strong teratogenic activities such as reduced average fetal weight (3.11±1.05; p<0.05), high resorbed litters numbers (3.78±5.09; p<0.05) as well as severe post implantation loss (31.80±40.41; p<0.05) were noted.
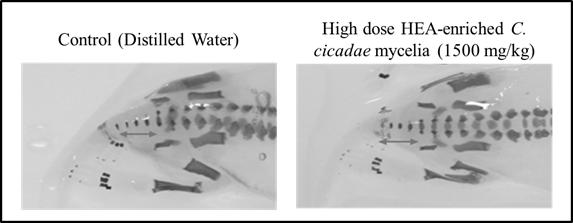
Lastly, based on the examination under the stereoscope, a total of 738 pups of the treated groups and 394 pups of the control groups showed no evidence of external and visceral variations (data not shown). Regarding fetal skeletal development, there were no statistically significant differences in the number of cervical vertebrae, thoracic vertebrae, lumbar, sacral vertebrae, caudal vertebrae, excessive fontanelle opening, fused ribs, sternum or sternal split between the control and high dose groups (Figure 3), illustrating that HEA-enriched C. cicadae mycelia has no teratogenic effect on the embryos of SD rats, even at the highest dose of 1,500 mg/kg bw.
A previous study indicated that C. cicadae has a substantially lower gene mutation rate than C. sinensis[29], demonstrating its stability in genomic structure and its capability in regulating secondary metabolite production. Based on this finding, C. cicadae can be expected as an herbal material with enormous development and usage prospects, covering food, healthcare, cosmetics, biological agricultural chemicals and medicines.
HEA-enriched C. cicadae mycelia was given to pregnant Sprague-Dawley rats via gavage during the critical period for early fetal structural development (day 6 to 15). Results showed no overt signs of reproductive or embryo-fetal developmental toxicity in the rats at the highest dose tested (1,500 mg/kg bw/day, the No Observed Effect Level). Based on these findings, it was considered that HEA-enriched C. cicadae mycelia revealed no teratogenicity in Sprague-Dawley rats.
I-Chen Li designed the experiment, analyzed the data, and wrote the first draft of the manuscript. Jui-Hsia Hsu and Ting-Wei Lin provided HEA-containing Cordyceps Cicadae mycelium for control and experimental groups. Wen-Hsin Lin assisted with the statistical analyses. Chin-Chu Chen coordinated the study, supervised the treatment, collected data, and helped write the manuscript.
The authors thank Hsin-Yun Yang for editing the manuscript.
Severity: Warning
Message: file_get_contents(): SSL operation failed with code 1. OpenSSL Error messages: error:14090086:SSL routines:ssl3_get_server_certificate:certificate verify failed
Filename: components/article-comp.php
Line Number: 630
Backtrace:
File: /home/siftdesk.org/public_html/application/views/components/article-comp.php
Line: 630
Function: file_get_contents
File: /home/siftdesk.org/public_html/application/views/components/article-details.php
Line: 70
Function: view
File: /home/siftdesk.org/public_html/application/views/article.php
Line: 7
Function: view
File: /home/siftdesk.org/public_html/application/controllers/Home.php
Line: 2262
Function: view
File: /home/siftdesk.org/public_html/index.php
Line: 316
Function: require_once
Severity: Warning
Message: file_get_contents(): Failed to enable crypto
Filename: components/article-comp.php
Line Number: 630
Backtrace:
File: /home/siftdesk.org/public_html/application/views/components/article-comp.php
Line: 630
Function: file_get_contents
File: /home/siftdesk.org/public_html/application/views/components/article-details.php
Line: 70
Function: view
File: /home/siftdesk.org/public_html/application/views/article.php
Line: 7
Function: view
File: /home/siftdesk.org/public_html/application/controllers/Home.php
Line: 2262
Function: view
File: /home/siftdesk.org/public_html/index.php
Line: 316
Function: require_once
Severity: Warning
Message: file_get_contents(https://www.siftdesk.org/articles/images/134/t1.html): failed to open stream: operation failed
Filename: components/article-comp.php
Line Number: 630
Backtrace:
File: /home/siftdesk.org/public_html/application/views/components/article-comp.php
Line: 630
Function: file_get_contents
File: /home/siftdesk.org/public_html/application/views/components/article-details.php
Line: 70
Function: view
File: /home/siftdesk.org/public_html/application/views/article.php
Line: 7
Function: view
File: /home/siftdesk.org/public_html/application/controllers/Home.php
Line: 2262
Function: view
File: /home/siftdesk.org/public_html/index.php
Line: 316
Function: require_once
Severity: Warning
Message: session_start(): open(/home/siftdesk.org/tmp/sess_46rlnoj7o1rqpqevtbopgrsehu, O_RDWR) failed: Disk quota exceeded (122)
Filename: controllers/Home.php
Line Number: 44
Backtrace:
File: /home/siftdesk.org/public_html/application/controllers/Home.php
Line: 44
Function: session_start
File: /home/siftdesk.org/public_html/index.php
Line: 316
Function: require_once
Severity: Warning
Message: session_start(): Failed to read session data: files (path: /home/siftdesk.org/tmp)
Filename: controllers/Home.php
Line Number: 44
Backtrace:
File: /home/siftdesk.org/public_html/application/controllers/Home.php
Line: 44
Function: session_start
File: /home/siftdesk.org/public_html/index.php
Line: 316
Function: require_once
