Deqiang Pu
Email: pdqpudeqiang@163.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 4
Page No: 654-660
Deqiang Pu
Email: pdqpudeqiang@163.com
Zhenlei Zheng1#, Hongling Liu1#, Xiaoqiang Wang2, Xinglong Wu1, Yu Chen1, Jili Deng1, Xuexin Chen3, Yuejian Li4, Deqiang Pu1,2,3*
1Institute of Plant Protection, Sichuan Academy of Agricultural Sciences, 20 Jingjusi Road, Chengdu 610066, China.
2Industrial Crop Research Institute, Sichuan Academy of Agricultural Sciences, 159 Huajin Road, Chengdu 610300, China.
3 Institute of Insect Sciences, Zhejiang University, Hangzhou 310058, China.
4 Vegetable Germplasm Innovation and Variety Improvement Key Laboratory of Sichuan Province, Horticulture Research Institute, Sichuan Academy of Agricultural Sciences, Chengdu 610066, China.
Norhayati Ngah(norhayatingah@unisza.edu.my)
Deqiang Pu, Development and reproduction of the hoverfly Eupeodes corollae (Diptera: Syrphidae)(2019)Journal of Earth Sciences & Environmental Studies 4(4)
Syrphidae is one of the most speciose families of true flies, with more than 6,100 described species and a worldwide distribution. They are important to humans because they act as crucial pollinators, biological control agents, decomposers and bioindicators. This study was conducted to determine the biology and behavior of Eupeodes corollae, which is an important pollinator and enemy of aphid, including prey consumption, mating behavior, oviposition, and predation of larvae, to provide a basis for breeding and in-depth study. Results indicated that adult life expectancies were 17.2 ± 1.2 days (n = 16) for males and 19.6 ± 1.3 days (n = 16) for females, average number of eggs laid by females was 799.2±49.9 (n=16) and the hatch rate was over 90%. Larval development required 7.9 ± 0.8 days (n = 40), and 997.9 ± 47.8 Aphis craccivora (n = 10) were consumed by each larva. Pupa lasted 7.2 ± 0.4 days (n = 40) in the root of the host plant, on the edge of the seedling basin, or 0.1–1.0 cm below the soil.
Key words: Eupeodes corollae, aphid, biocontrol
Syrphidae is one of the most diverse families in Diptera, with more than 6,000 described species [1-2]. They are usually referred as hoverflies and are the third-most speciose taxon in the Neotropical Region[3]. Their coloration ranges from orange brown in a few species to striking yellow and black patterns that cause them to be confused with bees and wasps (Hymenoptera). Adult hoverflies can hover on flowers, which can be used as mating sites and food sources (pollen and nectar). Therefore, the imagoes are considered as important pollinators of herbs. They are also considered as important group of resource insects because they have a great impact on controlling the population density of aphids, which are significant pests of forests, crops, vegetables, and flowers[4-8] . A third of Syrphidae larvae are predators that consume aphids and other Homopteran insects, and as such are important natural enemies of these pests. Syrphid species have also been used as bioindicators to assess biodiversity loss and the efficiency of restoration and conservation policies[9-12].
Eupeodes corollae (Fabricius) belongs to the Dipteranidae of Diptera, and E. corollae larvae feed on aphids and are one of the most important natural enemies that are used to control aphids[13]. Previous studies have documented the spawning and larval predation habits of mature E. corollae adults, as well as hoverfly feeding, mating, and spawning behaviors[14]. However, most scholars focus on the taxonomic and molecular studies of the hoverflies and some scholars have conducted selective studies on the spawning sites of the hoverflies[15-18]. This study aims to clarify the indoor biological characteristics of E. corollae and to establish the conditions that would make large-scale breeding and in-depth research possible.
E. corollae
Adults of E. corollae were collected from Chengxiang Town, Qingbaijiang District, Chengdu City, Sichuan Province and raised for more than five generations in the insect laboratory of Institute of Plant Protection, Sichuan Academy of Agricultural Sciences(Chengdu, Sichuan, China).
Devices
Rectangular net room (100 mesh; LWH: 150×120×150 cm), large rearing cage (1,000 mL; LWH: 17.5×12.0×6.5 cm), medium-sized nourishing cage (LWH: 60.0×50.0×50.0 cm), small nourishing cage (LWH: 30.0×30.0×30.0 cm), flower pot (dh: 16.5×10.5 cm), microscope (Leica S8 APD, Germany), 30× handheld magnifier, 9-cm Petri dish, 100-ml beaker, absorbent paper, 40 times handheld magnifier, black cardboard (LW: 25×40 cm), electronic timer (Loease) and fine brush were used in the study.
Cultivation of honey plants
Seeds of Coreopsis grandiflora Nutt. ex Chapm. were prepared for cultivation. Vermiculite, organic fertilizer and mushroom residue were used as the cultivation substrate, and the planting vector is a circular flower of Pots (dh:16.5×10.5 cm), 5-10 seeds per pot; seeded pots placed in net room (100 mesh, LWH: 150×120×150 cm), regular water replenishment until flowering. All our experients recordings were performed in 12:12 LD, using daylight fluorescent lamps (16 W/6500k ), unless otherwise stated. The temperature was 24.0℃ ± 1.0℃, similar to that of a temperate summer day when hoverflies tend to be active.
Breeding of E. corollae
Insects were reared in net room kept at 24.0 ± 1.0°C, 50% – 70% relative humidity, and 12-hour light and dark cycles with light intensities of 800–1270 Lux. The feeding system was based on broad bean seedlings as the host plant, supplemented by Aphis craccivora and Coreopsis grandiflora flower for larval and adults of E. corollae.
Mating behavior of E. corollae
Two pots of broad bean seedlings covered with Aphis craccivora were placed in a medium-sized insect cage with 30 pairs of newly emerged E. corollae. We recorded their mating behavior, mating place, and number of mating adults every two hours from 8:00–18:00, continuously for 5 days, we used an electronic timer to record the adult mating time.
Lifespan, fecundity and oviposition sites of E. corollae
Eighteen pairs of newly-emerged adults were placed in separate rearing boxes (1000 ml), in each box, two broad bean seedlings with length of 8–10 cm were placed in the box and each seeding moistened with wet cotton balls near the base end, these seedings were replaced with new bean seedlings every day at 9:30 am. For each replaced seeding, eggs oviposited on the back of the leaves, leaf sheaths, fronts of the leaves and stems were recorded until the female E. corollae died. Surviving adults in each box were recorded at 17:00 every day until all adults died.
Fecundity and larval predation of E. corollae
The newly grown eggs and the broad bean leaves of the hoverflies were placed in glass dishes (9.0 cm in diameter and 1.8 cm deep). The leaves were moistened with wet cotton balls and a leaf was placed in each glass dish. On the second day, observations were made under a stereomicroscope (Leica S8 APD, Germany) to observe whether the eggs had hatched. We selected neonate larvae (<12 h old) and placed them individually in small, round glass dishes (9.0 cm diameter and 1.8 cm deep). They were fed on a pure diet of Aphis craccivora at 24.0 ± 1.0°C and with a photoperiod of 14 h: 10 h (L: D)[19]. Eighty aphids were provided each day for days 1–3, 300 per day for days 4–7, 100 per day (with unarmed larvae added) for days 8–9, at which point they reached the pupate stage. The larvae were then transferred to the broad bean leaves with a fine brush. Each day, we recorded the remaining aphids at 10:00, until all the larvae of pupates were recorded.
Pupal development characteristics
We placed two pots of broad bean seedlings containing bean pods in the medium-sized insect-retaining cages with more than 100 larvae. The cages were moistened every day until the larvae became pupae. Between 10:00 and 17:00 every day, we recorded pupal location, external characteristics, soil depth, and number of emerged individuals.
Mating behavior of E. corollae
E. corollae generally lives on the flowers or leaves of the host plant. Males approach females from the back and stop on the female’s back to mate. During the mating process, females either fly away to escape mating or stop to accept mating. In the laboratory setting, mating time ranged from 1.3–3.2 h, with an average of 2.2 ± 0.5 h (n=60). Adults mated primarily between 10:00 and 14:00, when 72.1% of the mating took place (Figure 1). Mating behavior include: (1) male stopping at leaves or flowers to achieve a better line of sight and then chase the female when they identify an easier position from which to fly alongside the female; (2) males hovering in places where females often appear and initiate courtship once they found a female. Females do not actively seek males, and most of the time, females mate after feeding. Most mating adults rest on the flowers or stems of the broad bean seedlings, or at the bottom of the rearing cages to facilitate hiding and avoiding external disturbances (Figure 2).
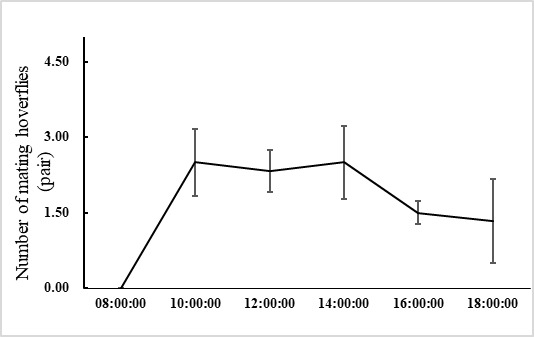
Fig. 1 Mating behavior of E. corollae at different time between 8:00 and 18:00, with peaks between 10:00 and 14:00.
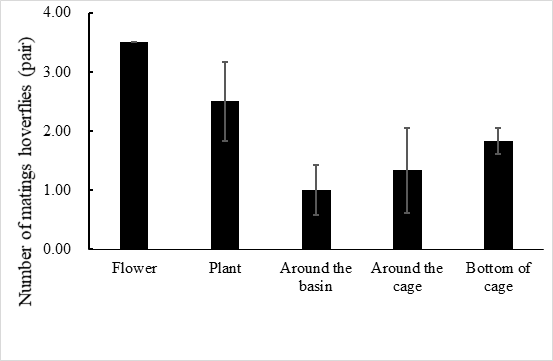
Fig. 2 Mating site of E. corollae in cages, the number of mating hoverflies are flower > plant > bottom of cage > around the cage > around the basin.
Lifespan, fecundity and spawning sites of adults
Before oviposition, females hover about 20 cm from the stem until they are close to an aphid swarm. The females walk several times around a small area of the plant with their ovipositor expanding and contracting and their antennae moving vertically. Finally, females elongate the oviposition tube and bent it toward the ventral surface. The oviposition process lasted 5 - 60 seconds, then females use their hind foot to clean their oviduct.
The lifespan of males ranges from 10 to 23 days with an average of 17.5 ± 1.2 days (n = 15) and the female lifespan ranged from 13 to 25 days with an average of 19.6 ± 1.3 (n = 15) days. More than 80% of the males survived more than 17.2 days, and same ratio of females survived more than 19.6 days (Figure 3). Adults begin to mate two days after hatching, oviposition begin four days after mating and the females laid eggs for 9 – 11 days until they die (Figure 4). Two oviposition peaks occurred: 7 and 18 days after emergence, with 77.7 ± 8.0 (n=16) and 67.4 ± 14.7 (n=16) eggs. The average of total egg production for per female was 799.2 ± 49.9 eggs (n=16), with a daily average of between 35 and 70 eggs. The average daily egg production volume fluctuates for each female and there is generally a 1–2 day interval where egg laying peak, with eight peaks occurring during the lifespan of each female. Females primarily oviposite on the back of the leaves, following by the leaf sheaths, and the fronts of the leaves, the stems were the least likely substrate for egg laying (Figure 5).
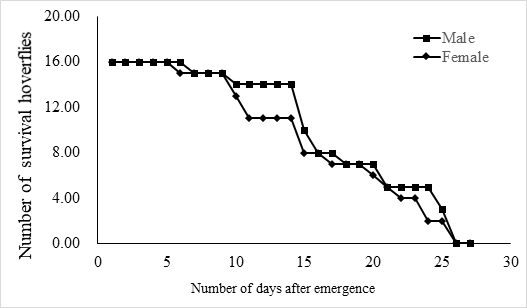
Fig.3 The life span of adult E. corollae. Males lived for 10–23 days, with an average of 17.5 ± 1.2 days (n = 16), and females for 13–25 days, with an average of 19.6 ± 1.3 days (n = 16).
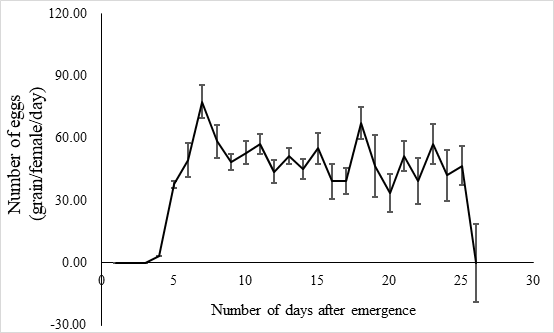
Fig. 4 Laying egg of E. corollae. The peak period of oviposition was 7 days (77.7±8.0 eggs) and 18 days (67.4±14.7 eggs) after emergence. Average egg production per female was 799.2 ± 49.9 (n=16), and the average daily egg production was 35.0 -70.0. The average daily egg production volume fluctuated for each female, and there was generally a 1-2-day interval where egg laying peaked, with eight peaks during the life of a female.
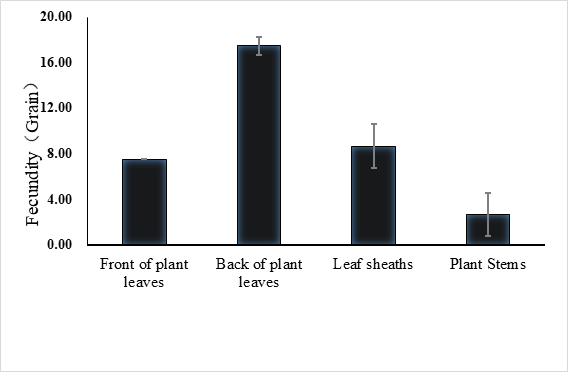
Fig. 5 Spawning site of E. corollae. females primarily oviposite on the back of the leaves, followed by the leaf sheaths and the fronts of the leaves, stems are the least likely substrate for egg laying.
Egg hatching and larval predation
The egg hatch rate reached over 92.5 % ± 0.6% (n=10) in the laboratory settings. The newly hatched larvae were 1.00 ± 0.03 mm (n = 40) long and covered with slightly fluffy hair. Larvae gradually became longer during the growth period, which lasted 7.9 ± 0.8 days (n = 40), each larva feeds 997.9 ± 99.8 A. craccivora (n=10). There was little aphid consumption by early instars before the third day, and with less than 80 aphids per day being attacked. Predatory behavior increased from the fourth day and peaks on the seventh day after hatching, when the number of aphids consumed by predators reach 307.7±6.5 (n=10) (Figure 6).
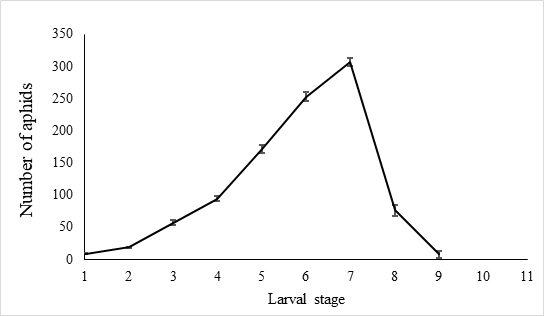
Fig. 6 Number of A. craccivora attacked by larval E. corollae. Predation among larvae was infrequent until the third day, with less than 80 aphids being attacked. Predatory behavior increased starting the fourth day and peaked seven days after hatching, when the number of aphids consumed by predators reached 307.7±6.5(n=10).
Pupal characteristics
Before pupation, the larvae reduce aphid intake and activity, and began to secrete a sticky exuvia substance around the leaves, stems, and seedling pots. After about 35 hours, the pupal surface hardened and there is a noticeable streak on the back. A crust gradually formed around the pupae, this process needs 4–10 hours. In the middle of the mid-palatosius, the characteristic stripe of the adult’s abdomen begins to appear. At the end of pupal development, the pupal shell become more and more transparent and the adult body color become darker, the pupal stage lasts for 7.2 ± 0.7 days (n = 40).
Results shew that E. corollae can develop and mate indoors under laboratory conditions, and the average fecundity of adults is 799.2±49.9 (n=16) (with a maximum of 1,410), which is consistent with previous study[20] , but not consistent with another report [21], which may be due to different fresh flowers, light, and temperature. Previous report stated that light has a certain degree of influence on the growth and development of E. corollae [14]. The egg hatch rate reached more than 90.0%, and the life span of males was 17.2 ± 1.2 days and 19.6 ± 1.3 days for females, which is similar to previous report (Dong 1988). According to another research [22], the daily maximum predation rates of 1–3 instar larvae on green peach aphids were 10.4 for the first instar, 55.7 for the second, and 166.7 for the third, our results show that the growth period of the larvae was 7.9 ± 0.8 days (n = 40), and the maximum predation amount occurred on the seventh day, with an average of 307.7 aphids were predated. We conclude that E. corollae is an important predator of aphids and that it could have practical value in aphid control the same as other results [23].
Predatory hoverfies, Coccinella septempunctata and Chrysopidae insect are important natural enemies of aphids, whiteflies and other agricultural and forestry pests. At the same time, hoverflies are important pollinators for cross-pollinated plants. Despite this, there is far less domestic research on the hoverfly than on ladybeetle and bees, the most important reason for this is that it is difficult for Syrphidae adults to oviposition their eggs in labs. Therefore, scholars mainly focus on the field protection and utilization of the Syrphidae flies. Among the natural enemies of aphids, aphid-eating flies are was one of the main predators[24], the ecology of E. corollae and its control of wheat aphids has been studied, results showed that an aphid reduction rate 80% was reached [25], and another study on the control of aphids on vegetables and chrysanthemums reported that an aphid reduction rate of 80% was also reached [26]. In recent years, scholars have done some research on the hoverfly as an important pollinating insect, in 2004, a study on the flower-visiting insect and pollinator, Paeonia lactiflora, showed that flies and bees demonstrated a high pollination intensity and efficiency [27]. Results about resources, population dynamics, and protection of the hoverfly in wheat fields showed that E. corollae was a dominant control Macrosiphum granarium species[28], almost all the literature has shown that the hoverfly has an important economic and ecological significance in pollination and in controlling pests. This study provides an important foundation for further understanding the pest control and pollination mechanisms of the hoverfly, moreover, it provides a more complete methodology for further exploration of artificial propagation techniques [29] and large-scale breeding for the scientific utilization of the flies that play an important role in pollination and in the control of aphid populations.
The E. corollae is an important natural enemy and pollinating insect. Through this study, the biological characteristics of the scorpion fly, such as adult life, spawning ability, larval predation ability and feeding technology system, have been clarified. Studying its control and pollination mechanisms lays the foundation.
This research was funded by Science & Technology of Sichuan Province (2018YYJC0468), National Research Program of China (2018YFD0201300) and National modern agricultural industry technology system Sichuan innovation team of tea to D. Q. P.
Brown BV. Introduction. In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado MA. (Eds) Manual of Central American Diptera, Vol. 1.NRC CNRC Research Press, Ottawa. 2009, 1 -7.
Qiao GX, Zhang GX, Simon JC, Dedryver CA, Rispe C and Hullé M. Preliminary study of aphid diversity in China: taxonomic and geographic variation.2004
Amorós-Jiménez R, Pineda A, Fereres A, and Marcos-García MÁ. Feeding preferences of the aphidophagous hoverfly Sphaerophoria rueppellii affect the performance of its offspring. Biocontrol. 2014,59, 427-435.
View ArticleSpeight MCD and Lucas JAW. Liechtenstei Syrphidae (Diptera). Berichte der Botanisch-Zoologischen Gesellschaft Liechtenstein-Sargans-Werdenberg. 1992, 19: 327 - 463.
Marinoni L and Thompson FC. Flower flies of southern Brazil (Diptera: Syrphidae). Part I. Introduction and new species. Studia Dipterologica. 2003, 10,565-578.
Huo KK, Zheng ZM and Zhang HJ. Present situation of researching on insects of Syrphidae in China. J. Hanzhong Teachers College (Nat Sci). 2002, 1, 70-75.
Ssymank A and Kearns C. In: Ssymank A, Hamm A, Vischer-Leopold M. (Eds) Caring for pollinators - safeguarding agro-biodiversity and wild plant diversity. Bundesamt für Naturschutz, Bonn., 2009, 39-52.
Inouye D, Larson B, Symank A and Kevan P. Flies and flowers III: ecology of foraging and pollination. J. Pollination Ecol. 2015, 16, 115-133. 15
View ArticleSommaggio D. Syrphidae: can they be used as environmental bioindicators.Agriculture, Ecosystems and Environment. , 1997, 74: 343-356. 00042-0
View ArticleTscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecology Letters. 2005, 8: 857-874.
View ArticlePérez-Bañón C, Juan A, Petanidou T, Marcos-García MA and Crespo MB.The reproductive ecology of Medicago citrina (Font Quer) Greuter (Leguminosae): a bee-pollinated plant in Mediterranean islands where bees are absent. Plant Syst. Evol. 2003. 241: 29-46.
View ArticleSommaggio D, Burgio G. The use of Syrphidae as functional bioindicator to compare vineyards with different managements. Bulletin of Insectology. 2014, 67(1): 147-156.
Wang B, Liu Y and Wang GR. Chemosensory genes in the antennal transcriptome of two syrphid species, Episyrphus balteatus and Eupeodes corollae (Diptera: Syrphidae). Bmc Genomics. 2017, 1:586. PMid:28784086
View Article PubMed/NCBIKarelin VD. Conditions for the use of syrphids. Zashchita Rastenii. 1980, 1, 40-41.
Marinoni L and Thompson FC. Flower flies of southern Brazil (Diptera: Syrphidae). Part I. Introduction and new species. Studia Dipterologica. 2003,10,565-578.
Wang B, Liu Y and Wang GR,. Chemosensory genes in the antennal transcriptome of two syrphid species, Episyrphus balteatus and Eupeodes corollae (Diptera: Syrphidae). Bmc Genomics. 2017, 1:586. PMid:28784086
View Article PubMed/NCBIXue BD, Gao GF and Wang WH.Controlling the Syrphid species and larvae control of soybean aphid in soybean field on the southwest slope of Changbai Mountains. J. Jilin Agric. Sci.2000, 25, 33-34.
Von Dohlen CD, Rowe CA and Heie OE. A test of morphologi-cal hypotheses for tribal and subtribal relationships of Aphidi-nae (Insecta: Hemiptera: Aphididae) using DNA sequences. Mol. Phylogene Evol. 2006, 38:316-329. PMid:16368250
View Article PubMed/NCBIShort BD and Bergh JC. Feeding and egg distribution studies of Heringia calcarata (Diptera: syrphidae), a specialized predator of woolly apple aphid (Homoptera: Eriosomatidae) in virginia apple orchards. J. Econ. Entomol.2004, 97, 813-819. PMid:15279258
View Article PubMed/NCBIDong HF and Xiong HZ. Rearing of the adults of Metasyrphus corollae (F.) (Dip:syrphidae),. Chin. J. Bio. 1988, 4, 155-158.
Dong K., Dong Y and Luo YZ. Studies on the indoor rearing of the hoverfly Syrphidae. Nat. Enemies Insects, 2004, 26, 97-100.
Li X Y and Luo YZ. Study on the predation of Syrphus corollae Fabricius to three aphids. J. Yunnan Agric. Univer. 2001, 16, 102-104.
He JL, Chu XP, Sun XQ and Ye WJ. Evaluation of feeding and utilization of hoverfly. J. Shanghai Jiaotong Univer. Agric. Sci. 1994, 2, 79-83.
Lu ZQ, Lin GL, Chen LF, Zhu L and Zhu SD. Studies on natural of aphids on vegetable crops and their control effects. Nat. Enemies insects.1986, 02, 63-71.
Yang FC, Li ZW, Gao HC, Ji FH and Lin GL. Boost and control effect on wheat aphid. Insect Respirator. 1989, 03, 116-121.
Sun XQ, He JL, Shen LJ, Li ZH and Ye WJ. Preliminary study on the control of aphids on vegetables and chrysanthemums using the hoverfly. J. Shanghai Agric College.1992, 01, 96-98 + 22.
Hong Y and Liu Q. Foraging and pollination insects of Paeonia lactiflora. Entomological Knowledge. 2004, 5: 449-454.
Jia XL, Li F and Li HZ. Research of the resources and population dynamics and protective utilization of Syrphus fabricius. J. Shanxi Agric. Sci. 2013, 41, 616-619.
Xu XN and Wang ED. Statue and analysis of overseas natural enemies merchandise. Chin. Biotechnol. 2007, 1, 373-382.
