Pathologic response to neoadjuvant treatment in locally advanced rectal cancer and impact on outcome
Introduction
Preoperative chemoradiotherapy (CRT) results in local control improvement and downstaging with, subsequently, the increased possibility of sphincter sparing in low rectal tumour and less toxicity in comparison with postoperative chemoradiation (1,2). Pathologic downstaging of a rectal cancer occurs when the final pathologic stage response is less than the preoperative stage of the tumour. A pathologic complete response (pCR) (absence of cancer cells in the resected surgical specimen) can occur after neoadjuvant treatment and this result may confer a survival advantage [overall survival (OS) and disease-free survival (DFS)] (3-12).
The aim of the current study is to assess the pathologic response rates following neoadjuvant CRT and evaluate the influence of response to neoadjuvant treatment on the long-term outcome in patients with locally advanced rectal cancer.
Methods
Patients
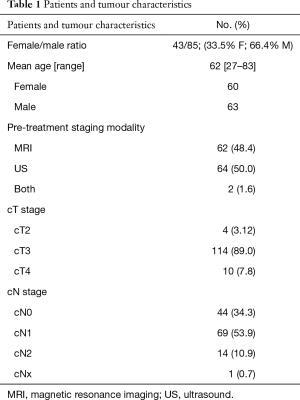
A total of 127 patients with locally advanced (clinical T3, T4 or node positive), biopsy proven adenocarcinoma of the rectum were involved in this retrospective review, with the mean age of 62.1 years (SD: 11.1 years) (ages ranged from 27–83 years). 33.85% (43 patients) were female and 66.15% (84 patients) male.
Pre-treatment staging was performed by endorectal ultrasound (ERUS) or magnetic resonance imaging (MRI), in addition to a range of other investigations including computed tomography (CT) of chest and abdomen, colonoscopy, complete blood count, blood chemistry and carcinoembriogenic antigen (CEA). All patients were treated at The Alfred Hospital, Melbourne, Australia, between 2005 and 2010.
Institutional review board approval was granted for this study.
Treatment
After signing informed consent forms, all patients received neoadjuvant radiotherapy, concurrent with 5-fluorouracil (5 FU), at the William Buckland Radiotherapy Centre (WBRC) at The Alfred Hospital. Patients received either 45 or 50.4 Gray (Gy) radiotherapy, 1.8 Gy per fraction, one fraction per day, and were treated 5 days per week. Treatment was delivered using a 3-field 18/6 MV photon technique on a lineal accelerator. The clinical target volume included the primary tumour and regional lymph nodes (mesorectal, presacral, internal iliac, obturator). All patients received either bolus or continue infusion 5 FU.
After radical surgery, all patients received adjuvant chemotherapy with weekly 379 mg per square meter 5 FU for 20 weeks.
Follow-up
Pathologic response was assessed by comparing postoperative pathologic staging (yp) with preoperative clinical staging and grouped as pathologic complete responders (pCR), partial responders (decrease in tumour or nodal staging) and non-responders.
During a median follow-up of 74 months, patients were clinically evaluated (history and examination) and were referred for radiological assessment (chest X-ray, abdominal-pelvic CT scan, colonoscopy and other investigations) as per clinical indications.
DFS was defined as the time between surgery and first recurrence (local or distant). Cancer specific survival was defined as the time between surgery and the time that cancer-related death occurred.
Statistical methods
Continuous data were expressed as mean (SD), median and the range between parentheses.
Qualitative data are presented as absolute numbers or percentages. Comparative analysis of the quantitative data was performed using the Student’s t-test. The chi-square or Fisher’s exact tests were used for comparing proportions as appropriate. Odds ratio (OR) and 95% confidence interval (CI) were reported for significant associations. Estimates of DFS and OS were calculated using the Kaplan-Meier method. Patient and disease factors were evaluated using log-ranked test, with result reported as median and 95% CI. Statistical significance was defined as P≤0.05.
Results
Patients and tumour characteristics
A total of 127 patients were involved in this study with the mean age of 62.1 years (SD: 11.1 years) (range, 27–83 years), 33.85% (43 patients) were female and 66.15% (84 patients) were male.
Fifty percent of the patients were staged by ERUS, 48.4% by MRI and 1.6% had both modalities for staging.
Patients and tumour characteristics are listed in Table 1.
Full table
Pathologic response
pCR was seen in 14.96% (19 patients) of participants. 58.26% (74 patients) of patients displayed partial response and TN staging did not change in 26.77% (34 patients). There was no significant association between patients and tumour characteristics and response to treatment (Table 2).
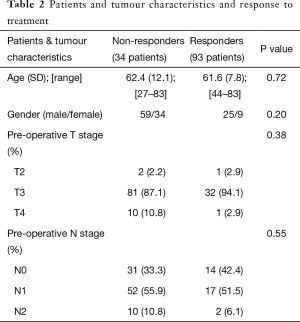
Full table
Treatment outcome
After a median follow-up of 74 months, 22 patients (17.32%) showed recurrence, including local and distant failure. Twenty patients developed distant metastases: six cases with liver metastases, six patients developed pulmonary metastases, one recurred in bone, two had pelvic recurrence and seven patients had metastases in more than one site (Table 3).
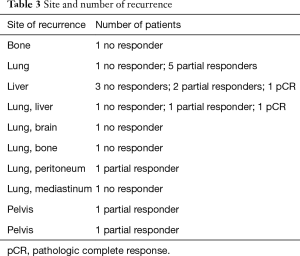
Full table
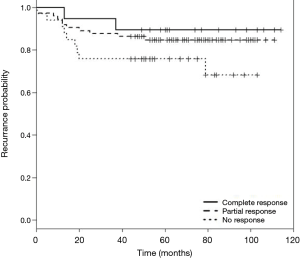
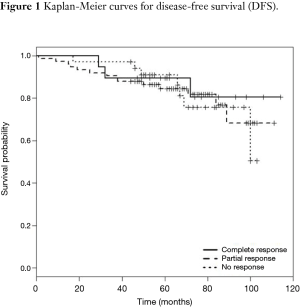
DFS and cancer-related survival were 82.67% and 88.97% respectively. DFS curve according to pathologic response is shown in Figure 1. OS curve according to pathologic response is shown in Figure 2.
Discussion
Neoadjuvant CRT before curative surgery is a widely adopted treatment method in locally advanced adenocarcinoma of rectum, especially in low and fixed rectal tumour, to improve resectability, tumour control and preserve anal sphincter.
Pathologic response to neoadjuvant treatment in locally advanced rectal adenocarcinoma may predict a survival advantage (DFS and OS) (2,3,5-7,13,14). pCR rates following preoperative CRT are shown in a body of research literature to range from 9% to 30% (1,2,7,8,10,11,15,16) while some studies report a partial response to neoadjuvant treatment of 51% (6,11). The correlation between response to neoadjuvant treatment and oncologic outcome has been reported in other studies (3,4,6-12,17-23).
To our knowledge, the data presented in this study is one of the largest single-institution reports in the Australian literature looking at response rates and long-term outcomes of patients with locally advanced, rectal adenocarcinoma.
In our study, 58.26% of the patients showed some response to preoperative treatment. pCR was found in 14.96% of patients, which is comparable to European studies which reported rates of 11–16% (1,2,16). None of our patients with complete response to neoadjuvant treatment showed local recurrence. A lower rate of distant failure was observed in the pCR group compared with the other two groups and partial responders had less recurrence than non-responders.
DFS and OS in the pCR group were 89.47% and 94.7% respectively.
In partial responders, DFS and OS were 85.1% and 90.5% and in non-responders were 73.5% and 82.3% respectively. Table 4 demonstrates recurrence and cancer-related death of the three groups of responders.

Full table
Conclusions
This study revealed that preoperative chemoradiation for locally advanced adenocarcinoma of the rectum resulted in a pathologic response of the primary tumour and lymph nodes in the majority of patients studied. Our results indicate pCR is achievable in a proportion of patients and that response to pre-operative CRT can be used as a predictor of tumour recurrence rate and long-term outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board (No. 218/12).
References
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620-5. [Crossref] [PubMed]
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688-96. [Crossref] [PubMed]
- Berger C, de Muret A, Garaud P, et al. Preoperative radiotherapy (RT) for rectal cancer: predictive factors of tumor downstaging and residual tumor cell density (RTCD): prognostic implications. Int J Radiat Oncol Biol Phys 1997;37:619-27. [Crossref] [PubMed]
- Janjan NA, Crane C, Feig BW, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol 2001;24:107-12. [Crossref] [PubMed]
- Theodoropoulos G, Wise WE, Padmanabhan A, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum 2002;45:895-903. [Crossref] [PubMed]
- Rosenberg R, Nekarda H, Zimmermann F, et al. Histopathological response after preoperative radiochemotherapy in rectal carcinoma is associated with improved overall survival. J Surg Oncol 2008;97:8-13. [Crossref] [PubMed]
- Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys 2002;53:664-74. [Crossref] [PubMed]
- Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 2008;72:99-107. [Crossref] [PubMed]
- Ciccocioppo A, Stephens JH, Hewett PJ, et al. Complete pathologic response after preoperative rectal cancer chemoradiotherapy. ANZ J Surg 2009;79:481-4. [Crossref] [PubMed]
- Biondo S, Navarro M, Marti-Rague J, et al. Response to neoadjuvant therapy for rectal cancer: influence on long-term results. Colorectal Dis 2005;7:472-9. [Crossref] [PubMed]
- García-Aguilar J, Hernandez de Anda E, Sirivongs P, et al. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum 2003;46:298-304. [Crossref] [PubMed]
- Kuo LJ, Liu MC, Jian JJ, et al. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol 2007;14:2766-72. [Crossref] [PubMed]
- Benzoni E, Intersimone D, Terrosu G, et al. Prognostic value of tumour regression grading and depth of neoplastic infiltration within the perirectal fat after combined neoadjuvant chemo-radiotherapy and surgery for rectal cancer. J Clin Pathol 2006;59:505-12. [Crossref] [PubMed]
- Lee JH, Kim SH, Kim JG, et al. Preoperative chemoradiotherapy (CRT) followed by laparoscopic surgery for rectal cancer: predictors of the tumor response and the long-term oncologic outcomes. Int J Radiat Oncol Biol Phys 2011;81:431-8. [Crossref] [PubMed]
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23. [Crossref] [PubMed]
- Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012;30:1770-6. [Crossref] [PubMed]
- Janjan NA, Khoo VS, Abbruzzese J, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 1999;44:1027-38. [Crossref] [PubMed]
- Onaitis MW, Noone RB, Hartwig M, et al. Neoadjuvant chemoradiation for rectal cancer: analysis of clinical outcomes from a 13-year institutional experience. Ann Surg 2001;233:778-85. [Crossref] [PubMed]
- Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg 2002;236:75-81. [Crossref] [PubMed]
- Vecchio FM, Valentini V, Minsky BD, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005;62:752-60. [Crossref] [PubMed]
- Quah HM, Chou JF, Gonen M, et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008;113:57-64. [Crossref] [PubMed]
- Kim NK, Baik SH, Seong JS, et al. Oncologic outcomes after neoadjuvant chemoradiation followed by curative resection with tumor-specific mesorectal excision for fixed locally advanced rectal cancer: Impact of postirradiated pathologic downstaging on local recurrence and survival. Ann Surg 2006;244:1024-30. [Crossref] [PubMed]