Abstract
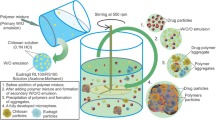
Gliclazide is a second generation of hypoglycemic sulfonylurea and acts selectively on pancreatic β cell to control diabetes mellitus. The objective of this study was to produce a controlled release system of gliclazide using chitosan beads. Chitosan beads were produced by dispersion technique using tripolyphosphate (TPP) as gelating agent. The effects of process variables including chitosan molecular weight, concentration of chitosan and TPP, pH of TPP, and cross-linking time after addition of chitosan were evaluated by Taguchi design on the rate of drug release, mean release time (MRT), release efficiency (RE8%), and particle size of the beads. The blood glucose lowering effect of the beads was studied in normal and streptozotocin-diabetic rats. The optimized formulation CL2T5P2t10 with about 31% drug loading, 2.4 h MRT, and 69.16% RE8% decreased blood glucose level in normal rats for 24 h compared to pure powder of gliclazide that lasted for just 10 h.





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
J. A. K. Lauwo, D. K. Agrawal, and I. V. Emenike. Some pharmaceutical studies on sustained release coprecipitates of ampicillin-trihydrate with acrylic resin (Eudragit®-RS). Drug Dev. Ind. Pharm. 16:1375–1389 (1990).
R. Bodmeier, H. Chen, P. Tyle, and P. Jarosz. Pseudophedrine HCL microspheres formulated into an oral suspension dosage form. J. Control. Release. 15:65–77 (1991).
A. K. Anal, W. F. Stevens, and C. Remuñán-López. Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int. J. Pharm. 312(1–2):166–173 (2006).
I. M. van der Lubben, J. C. Verhoef, G. Borchard, and H. E. Junginger. Chitosan for mucosal vaccination. Adv. Drug Deliv. Rev. 52:139–144 (2001).
A. D. Martino, M. Sittinger, and M. V. Risbud. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 26:5983–5990 (2005).
L. Y. Wang, Y. H. Gu, Q. Z. Zhou, G. H. Ma, Y. H. Wan, and Z. G. Su. Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf., B: Biointerfaces. 50:126–135 (2006).
J. Wu, W. Wei, L. Y. Wang, Z. G. Su, and G. H. Ma. Preparation of uniform-sized pH-sensitive quaternized chitosan microsphere by combining membrane emulsification technique and thermal-gelation method. Colloids Surf., B: Biointerfaces. 63(2):164–175 (2008).
R. Bodmeier, K. H. Oh, and Y. Pramar. Preparation and evaluation of drug-containing chitosan beads. Drug Dev. Ind. Pharm. 15:1475–1494 (1989).
A. D. Sezer, and J. Akbûga. Release characteristics of chitosan treated alginate beads. I. Sustained release of a macromolecular drug from chitosan treated alginate beads. J. Microencap. 16(2):195–203 (1999).
A. K. Anal, D. Bhopatkar, S. Tokura, H. Tamura, and W. F. Stevens. Chitosan-alginate multilayer beads for gastric passage and controlled intestinal release of protein. Drug Dev. Ind. Pharm. 29:713–724 (2003).
A. K. Anal, and W. F. Stevens. Chitosan–alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 290:45–54 (2005).
D. Bhopatkar, A. K. Anal, and W. F. Stevens. Ionotropic alginate beads for controlled intestinal protein delivery: Effect of chitosan and barium counterions on entrapment and release. J. Microencap. 22:91–100 (2005).
C. Remuñán-López, and R. Bodmeier. Effect of formulation and process variables on the formation of chitosan–gelatin coacervates. Int. J. Pharm. 135:63–72 (1996).
R. O. Williams III, M. K. Barron, M. J. Alonso, and C. Remuñán-López. Investigation of a pMDI formulation containing chitosan microspheres. Int. J. Pharm. 174:209–222 (1998).
P. He, S. S. Davis, and L. Illum. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 187:53–65 (1999).
C. Remuñán-López, M. L. Lorenzo-Lamosa, J. L. Vila-Jato, and M. J. Alonso. Development of new chitosan–cellulose multicore microparticles for controlled drug delivery. Eur. J. Pharma. Biopharm. 45:49–56 (1998).
F. L. Mi, S. S. Shyu, T. Wong, S. F. Jang, S. T. Lee, and K. T. Lu. Chitosan–polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. II. Effect of pH-dependent ionic cross-linking or interpolymer complex using tripolyphosphate or polyphosphate reagent. J. Appl. Polym. Sci. 74:1093–1107 (1999).
J. A. Ko, H. J. Park, S. J. Hwang, J. B. Park, and J. S. Lee. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 249(1–2):165–174 (2002).
F. L. Mi, S. S. Shyu, C. T. Chen, and J. Y. Schoung. Porous chitosan microsphere for controlling the antigen release of Newcastle disease vaccine: Preparation of antigen-adsorbed microsphere and in vitro release. Biomaterials. 20(17):1603–1612 (1999).
S. Nakai, K. Koide, and K. Eugster. A new mapping super-simplex optimization for food product and process development. J. Food Sci. 49:1143–1170 (1984).
K. C. Chen, T. C. Lee, and J. Y. Houng. Search method for the optimal medium for the production of lactase by Kluyveromyces fragilis. Enzyme Microb. Technol. 14:659–664 (1992).
R. Banerjii, and B. C. Bhattacharyya. Evolutionary operation (EVOP) to optimize three-dimensional biological experiments. Biotechnol. Bioeng. 41:67–71 (1993).
R. Tunga, R. Banerjii, and B. C. Bhattacharyya. Optimization of n variable biological experiments by evolutionary operation-fractional design technique. J. Biosci. Bioeng. 87(2):224–230 (1999).
J. Y. Houng, K. C. Chen, and W. H. Hsu. Optimization of cultivation medium composition for isoamylase production. Appl. Microb. Biotechnol. 31:61–64 (1989).
S. Chopra, G. V. Patil, and S. K. Motwani. Release modulating hydrophilic matrix systems of losartan potassium: Optimization of formulation using statistical experimental design. Eur. J. Pharm. Biopharm. 66:73–82 (2007).
O. Sreekumar, N. Chand, and A. C. Basappa. Optimization and interaction of media components in ethanol production using Zymomonas mobilis by response surface methodology. J. Biosci. Bioeng. 88(3):334–338 (1999).
M. Jahanshahi, G. Najafpour, and M. Rahimnejad. Applying the Taguchi method for optimized fabrication of bovine serum albumin (BSA) nanoparticles as drug delivery vehicles. Afr. J. Biotechnol. 7(4):362–367 (2008).
B. D. Cobb, and J. M. Clarkson. A simple procedure for optimising the polymerase chain reaction (PCR) using modified Taguchi methods. Nucleic Acids Res. 22(18):3801–3805 (1994).
R. A. Stone, and A. Veevers. The Taguchi influence on designed experiments. J. Chemometr. 8:103–110 (1994).
J. D. Bendell, J. Disney, and W. A. Pridmore. Taguchi Methods: Applications in World Industry, IFS Publications, Bedford, 1989.
J. Y. Houng, H. F. Hsu, Y. H. Liu, and J. Y. Wu. Applying the Taguchi robust design to the optimization of the asymmetric reduction of ethyl 4-chloro acetoacetate by bakers’ yeast. J. Biotechnol. 100(3):239–250 (2003).
F. K. Główka, T. W. Hermann, and M. Zabel. Bioavailability of gliclazide from some formulation tablets. Int. J. Pharm. 172(1–2):71–77 (1998).
D. B. Cambell, R. Lavielle, and C. Nathan. The mode of action and clinical pharmacology of gliclazide: A review. Diabetes Res. 14(Suppl):S21–S36 (1991).
European Diabetes Policy Group. A desktop guide to type 2 diabetes mellitus. Diabet. Med. 16:716–730 (1999).
A. Harrower. Gliclazide modified release: From once daily administration to 24-hour blood glucose control. Metabolism. 49(suppl 2):7–11 (2000).
M. Francillard, N. Frey, and M. Paraire. Pharmacokinetics of diamicron modified release (MR) in 1007 type 2 diabetic patients. J. Nutr. Health Aging. 5:31 (2001).
P. Delrat, M. Paraire, and R. Jochemsen. Complete bioavailability and lack of food effect on pharmacokinetics of gliclazide 30 mg modified release in healthy volunteers. Biopharm. Drug Dispos. 23:151–157 (2002).
F. M. Gribble, and F. M. Ashcroft. Differential sensitivity of β-cell and extrapancreatic K ATP channels to gliclazide. Diabetologia. 42:845–848 (1999).
F. M. Gribble, S. J. Tucker, and S. Seino. Tissue specificity of sulfonylureas: Studies on cloned cardiac and β cell KATP channels. Diabetes. 47:1412–1418 (1998).
G. Schernthaner. Gliclazide modified release: a critical review of pharmacodynamic, metabolic, and vasoprotective effects. Metabolism. 52(Suppl 1):29–34 (2003).
G. Taguchi. System of Experimental Design. Engineering Methods to Optimize Quality and Minimize Costs, Kraus International, White Plains, New York, 1987.
W. Y. Fowlkes, and C. M. Creveling. Engineering Methods for Robust Product Design. Using Taguchi Methods® in Technology and Product Development, Addison-Wesley, New York, 1995.
W. C. Lin, D. G. Yu, and M. C. Yang. pH-sensitive polyelectrolyte complex gel microspheres composed of chitosan/sodium tripolyphosphate/dextran sulfate: Swelling kinetics and drug delivery properties. Colloids Surf., B: Biointerfaces. 44(2–3):143–151 (2005).
J. A. Ko, H. J. Park, S. J. Hwang, J. B. Park, and J. S. Lee. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 249(1–2):165–174 (2002).
P. Costa, J. Manuel, and S. Lobo. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 13:123–133 (2001).
M. C. Gohel, and M. K. Panchal. Novel use of similarity factors f 2 and S d for development of diltiazem HCl modified-release tablets using a 32 factorial design. Drug Dev. Ind. Pharm. 28(1):77–87 (2002).
A. Eidi, M. Eidi, and M. Sokhteh. Effect of fenugreek (Trigonella foenum-graecum L) seeds on serum parameters in normal and streptozotocin-induced diabetic rats. Nutr. Res. 27(11):728–733 (2007).
G. Suresh Kumar, A. K. Shetty, K. Sambaiah, and P. V. Salimath. Antidiabetic property of fenugreek seed mucilage and spent turmeric in streptozotocin-induced diabetic rats. Nutr. Res. 25(11):1021–1028 (2005).
A. Chandra, A. A. Mahdi, S. Ahmad, and R. K. Singh. Indian herbs result in hypoglycemic responses in streptozotocin-induced diabetic rats. Nutr. Res. 27(3):161–168 (2007).
N. Pulido, A. Suarez, B. Casanova, R. Romero, E. Rodriguez, and A. Rovira. Gliclazide treatment of streptozotocin diabetic rats restores GLUT4 protein content and basal glucose uptake in skeletal muscle. Metabolism. 46(Suppl 1):10–13 (1997).
X. Qiang, J. Satoh, M. Sagara, M. Fukuzawa, T. Masuda, S. Miyaguchi, K. Takahashi, and T. Toyota. Gliclazide inhibits diabetic neuropathy irrespective of blood glucose levels in streptozotocin-induced diabetic rats. Metabolism. 47(8):977–981 (1998).
K. J. Palmer, and R. N. Brogden. Gliclazide, an update of its pharmacological properties and therapeutic efficacy non-insulin-dependent diabetes mellitus. Drugs. 46:92–125 (1993).
J. Duhault, M. Boulanger, and F. Tisserand. The pharmacology of S 1702, a new highly effective oral antidiabetic drug with unusual properties. Arzneim Forsch. 22:1682–1685 (1972).
A. E. Pontiroli, A. Calderara, and G. Pozza. Secondary failure of oral hypoglycaemic agents: Frequency, possible causes, and management. Diabetes Metab. Rev. 10:31–43 (1994).
Acknowledgments
The authors wish to acknowledge the Vice Chancellor of Research of Isfahan University of Medical Sciences for the financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varshosaz, J., Tavakoli, N., Minayian, M. et al. Applying the Taguchi Design for Optimized Formulation of Sustained Release Gliclazide Chitosan Beads: An In Vitro/In Vivo Study. AAPS PharmSciTech 10, 158–165 (2009). https://doi.org/10.1208/s12249-009-9191-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9191-8
Key words
Profiles
- J. Varshosaz View author profile