Abstract
Growing interest in developing devices that can be implantable or wearable requires the identification of suitable materials for the components of these devices. Electrochemical supercapacitors are not the exception in this trend, and identifying electrode materials that can be not only suitable for the capacitive device but also biocompatible at the same time is important. In addition, it would be advantageous if physiological fluids could be used instead of more conventional (and often corrosive) electrolytes for implantable or wearable supercapacitors. In this study, we assess the biocompatibility of films of anodized TiO2 nanotubes subjected to the subsequent annealing in Ar atmosphere and evaluate their capacitive performance in a physiological liquid. A biocompatibility test tracking cell proliferation on TiO2 nanotube electrodes and electrochemical tests in 0.01 M phosphate-buffered saline solution are discussed. It is expected that the study will stimulate further developments in this area.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Electrochemical capacitors (ECs), also called supercapacitors, are promising candidates for electrical energy storage devices with characteristics intermediate between traditional capacitors and batteries.1,2 ECs are mainly based on two energy storage mechanisms which represent a non-faradaic process and faradaic processes. The non-faradaic process includes physical adsorption/desorption on the electrolyte/electrode interface. The negative electrode attracts cations of an electrolyte and the positive electrode attracts anions during the charging process to form two electric double layers on two electrode/electrolyte interfaces; the cations and anions are then released back to electrolyte when the EC discharges. This type of ECs is commonly called electrochemical double-layer capacitors (EDLCs) and their electrodes are typically high surface area carbon materials.3–6 The faradaic processes are similar to mechanisms in batteries as reversible redox reactions are involved. In supercapacitors, the redox reactions typically occur only on the surface of electrodes. This type of supercapacitors based on the redox processes in the electrodes is also commonly called pseudocapactors.7 RuO2 is a classic example of an electrode material for supercapacitors operating via a pseudocapacitive (redox) mechanism.1,7–10
TiO2 is an interesting electrode materials for the state-of-the-art electrochemical energy storage systems and can be used both lithium-ion batteries11–13 and electrochemical supercapacitors.12 Films of TiO2 nanotube arrays have particularly attracted attentions for the application in supercpacitors because of their high surface area and have been a subject of a number of studies.14–28 Based on the ideal capacitive response of TiO2 (rectangular cyclic voltammetry curves) observed in some cases, researchers have previously assigned the storage mechanism in TiO2 nanotube electrodes predominantly to the conventional electric double layer storage (see, for example,17,23). Due to the low conductivity of TiO2 and the amorphous nature of the anodized nanotubes, the capacitance of the pristine, as-anodized layer of TiO2 nanotubes is quite low and, therefore, a number of approaches have been developed to improve the capacitance and rate capability of TiO2 nanotube arrays via post-synthesis treatment and modification of its electronic conductivity, Ti3+/Ti4+ ratio, hydrogenation and induction of oxygen vacancies. For example, annealing in Ar atmosphere,14,21–23 thermal treatment in H2 atmosphere,15,19 annealing in NH3 atmosphere,16 cathodic biasing of TiO2 in an ethylene glycol electrolyte,18 second anodization with post-annealing in vacuum,20 electrochemical hydrogenation or plasma-treatment,24,25 and cathodic polarization treatment.27,28 Capacitances between 1 and 20 mF/cm2 have been commonly reported after conducting these additional treatments.
New emerging applications of electrochemical supercapacitors include ECs as power sources for implantable and wearable devices (such as, for example, pacemakers, implanted chips, sensors, smart power bodysuits).29,30 For such applications it is advantageous to use biocompatible electrode materials and other components of supercapacitors. It is also beneficial to shift from conventional electrolytes (often corrosive) to the electrolyte resembling physiological fluids.29 It is also important to note that inorganic electrode materials in implantable or wearable supercapacitors have an advantage of possibly higher volumetric energy and power densities, and therefore may be preferred to carbon materials. In this article, biocompatibility and the possibility of operation in a physiological-type electrolyte are investigated for the anodized films of titanium dioxide nanotubes post-annealed in Ar atmosphere. The phase composition and morphology of the nanotubes are assessed. Their encouraging electrochemical performance in neutral aqueous electrolytes (such as 3 M KCl) as well as in a phosphate-buffered saline is presented. The phosphate-buffered saline is a common buffer solution used in biological research, non-toxic for cells (the consequences of leakage of such an electrolyte from a wearable or implantable device would be dramatically milder than for the leakage of conventional electrolytes). It is shown that the nanotube films have a suitable capacitive behavior in that type of electrolyte. The results of the biocompatibility test of the TiO2 nanotube films are also reported.
Experimental
Titanium foils (purity of 99.7%) were used for anodization, which was performed in accordance with the procedure outlined elsewhere.31 A two-electrode electrochemical system was used at room temperature. The electrolyte was a mixture of glycerol, 0.5 wt% NH4F and 20 vol% H2O. A Ti foil was used as a working electrode in each anodization experiment and a Pt foil was used as a counter electrode. The two electrodes were separated by a distance of 30 mm. A direct current power supply was used to control the voltage. The titanium foils were anodized at 30 V for 2 h to produce films of TiO2 nanotubes on Ti foils. Three nanotube samples were subsequently annealed at 600°C, 650°C and 700°C in Ar atmosphere for one hour. The three types of sample used in the tests are described in Table I.
Table I. Sample Description.
| Sample Name | Description |
|---|---|
| NT-600 | Anodized nanotubes annealed at 600°C for one hour in Ar atmosphere |
| NT-650 | Anodized nanotubes annealed at 650°C for one hour in Ar atmosphere |
| NT-700 | Anodized nanotubes annealed at 700°C for one hour in Ar atmosphere |
X-ray diffraction (XRD, PANalytical X-pert Pro MRD XL with Cu Kα radiation (λ = 1.5418 Å)) was used to analyze the phase composition of the samples. The scan rate and step angle used for the measurements were 2 s/step and 0.02°, respectively, and the measurements were performed over a range of 2θ from 20° to 50°. The HighScore Plus v.3.0x software (PANalytical B.V. Almelo, The Netherlands) was used to analyze the recorded data. The surface morphology of titanium dioxide nanotubes was observed by scanning electron microscopy (SEM, Carl Zeiss SUPRA 55VP instrument).
The electrochemical properties were measured in the three-electrode cell including a working electrode (TiO2 nanotubes on Ti foil), a counter electrode (Pt wire) and a reference electrode (Ag/AgCl). The cells were filled with 3 M KCl solution or 0.01 M phosphate-buffered saline (PBS, pH 7.4, Sigma-Aldrich) solution under vacuum. The electrochemical properties were characterized by galvanostatic charge-discharge and cyclic voltammetry (CV) measurements using a Solartron Analytical 1470E instrument. The potential window between −0.1 and 0.6 V vs Ag/AgCl was used in tests. The CV plots were recorded at scan rates from 1 to 500 mV/s. Galvanostatic charge-discharge curves were recorded at various current densities between 1.5 to 10 μA/cm2 (per square cm of Ti substrate). The electrochemical impedance spectroscopy (EIS) was performed at the open circuit potential (OCP) within a frequency range from 100 kHz to 0.01 Hz using an Ivium-n-stat electrochemical analyzer. The amplitude of the modulation signal was set to 5 mV. Experimental data were fitted with ZView v.3.1 software (Scribner Associates, Inc., UK).
The biocompatibility of all samples was evaluated using Osteoblast-like cells (SaOs-2, Sarcoma osteogenic) (Barwon Biomedical Research, Geelong Hospital, Victoria, Australia), a human osteosarcoma cell line.32,33 All samples for cell culture were sterilized in a muffle furnace at 180°C for 1 h. The samples were placed in a well in a 24-well cell culture plate. SaOs-2 cells were seeded on the surface of samples and the control without any sample at a cell density of 5 × 103 cells per well. MTS assay was used to measure the in vitro proliferation of the SaOs-2 cells after cell culture for 7 days. The control is considered as biocompatible. In all cases, one-way analysis of variance was employed to evaluate the significant difference in the data, and the statistical difference was thought to be significant at p < 0.05.
Results and Discussion
Characterization of nanotube films
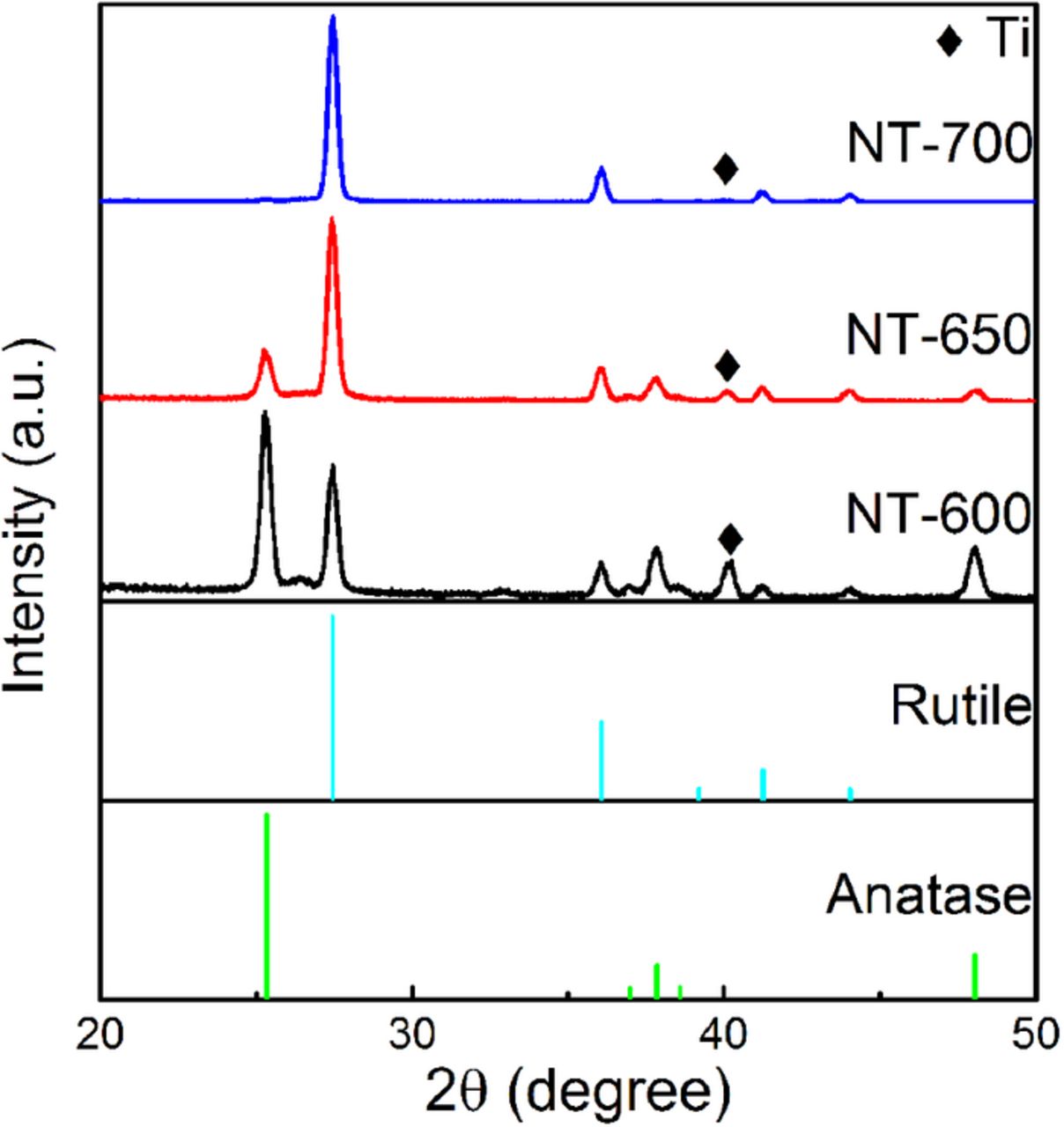
Figure 1 shows the XRD patterns of TiO2 nanotube films annealed at 600, 650 and 700°C for one hour. As it can be seen in Figure 1, XRD patterns of all samples have main peaks that match either rutile or anatase phases of TiO2. The peaks located at 25.3°, 36.9°, 37.8°, 38.5° and 48° match the standard diffraction file of the anatase TiO2 phase (# 01-070-7348); the peaks located at 27.4°, 36°, 39.1°, 41.2° and 44° match the standard diffraction file of the rutile TiO2 phase (# 01-072-1148); finally, the peak at 40.1° matches the strongest peak of the Ti phase (Powder Diffraction File # 03-065-6231). It can be seen that the increase in annealing temperature leads to the formation of larger amounts of the rutile phase in the samples because more of the anatase phase transforms to the more stable rutile phase at higher temperatures. At 700°C, the anatase phase almost disappears and the pattern is dominated by the peaks of rutile TiO2.
Figure 1. XRD patterns of the samples (NT-600, NT-650 and NT-700).
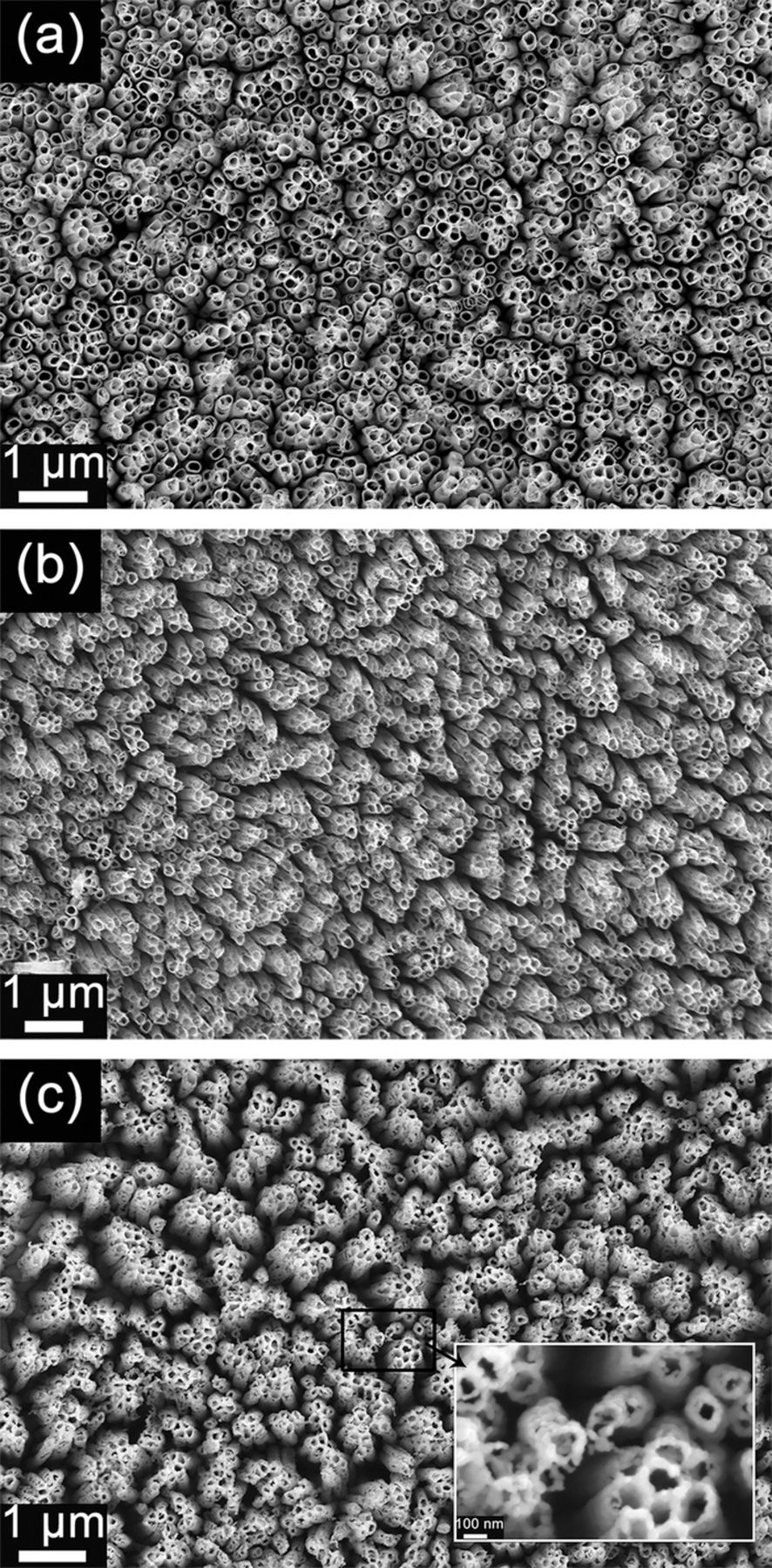
According to the SEM images in Figure 2a, 2b, 2c, the typical morphologies of structures in the TiO2 films on Ti can be observed. Arrays of nanotubes can be seen for each sample. The morphology changes after annealing at a higher temperature, and the nanotubes form bundles with increased gaps between the bundles. An enlarged SEM image of the sample NT-700 is shown in the inset of Figure 2c. It can be concluded the microstructure of the film changes when the annealing temperature is increased.
Figure 2. SEM images of the TiO2 nanotube films: (a) NT-600, (b) NT-650, (c) NT-700. The inset shows a higher magnification image of the nanotube layer in NT-700.
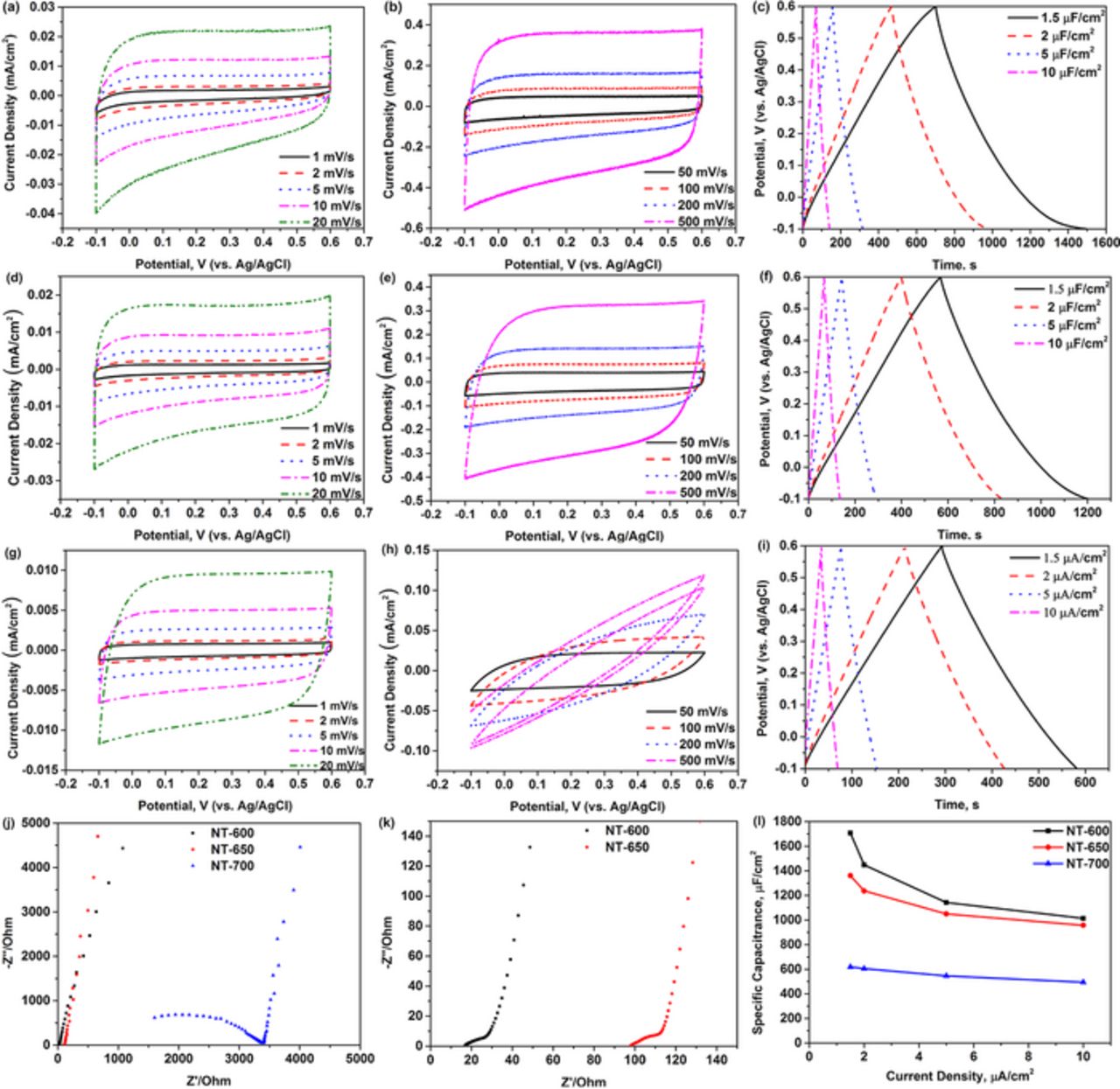
The electrochemical properties of the three samples of nanotube films (NT-600, NT-650 & NT-700) in 3 M KCl aqueous electrolyte are shown in Figure 3. It can be seen in Figure 3a, 3d, 3g that the cyclic voltammetry (CV) curves of the three samples recorded at slow scan rates (in the range between 1 and 20 mV/s) in a three-electrode system have an ideal rectangular shape, mirror-symmetric in respect to the horizontal axis. The CV curves of NT-600 and NT-650 remain close to ideal and mirror-symmetric at higher scan rates (50 to 500 mV/s), as shown in Figure 3b, 3e. In contrast, the CV curves of the sample NT-700 become elliptical at high scan rates (100 to 500 mV/s), as it can be seen in Figure 3h. It appears that the sample NT-700 deviates from the ideal capacitive behavior at high scan rates.
Figure 3. Electrochemical properties of NT-600, NT-650 and NT-700 in 3 M KCl aqueous electrolyte: (a, b and c) cyclic voltammetry and galvanostatic charge-discharge curves of titanium dioxide nanotubes NT-600; (d, e and f) cyclic voltammetry and galvanostatic discharge-charge curves of NT-650; (g, h and i) cyclic voltammetry and galvanostatic discharge-charge curves of NT-700; (j) electrochemical impedance spectra of the three samples; (k) enlarged view of the impedance spectra for NT-600 and NT-650; (l) the plot of capacitance as a function of current density for the three samples.
The galvanostatic charge-discharge curves of the three samples recorded at various current densities (1.5, 2, 5 and 10 μA/cm2) are shown in Figure 3c, 3f, 3i. The shapes of all charge-discharge curves for the three samples are almost ideally triangular. The capacitances per unit of the electrode surface of NT-600, NT-650 and NT-700 can be calculated from the formula:
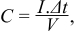
where C is the capacitance per unit of the electrode surface (μF/cm2), I is the current density per unit of the electrode surface (μA/cm2), Δt is the time of discharge (s), and V is the potential window (V). The capacitances of NT-600 and NT-650 can be up to 1700 μF/cm2 and 1360 μF/cm2, respectively, at a current density of 1.5 μA/cm2. The sample NT-700 shows a sharply lower capacitance of only 620 μF/cm2 at the same low current density.
The electronic conductivities of the three samples were evaluated by electrochemical impedance spectroscopy, and the Nyquist plots are shown in Figure 3j. The semi-circle area of plots for NT-600 and NT-650 is enlarged in Figure 3k. The diameter of the semi-circle corresponds to the so called charge-transfer resistance at the electrode-electrolyte interface, which is often correlated with the electronic conductivity of an electrode. The semi-circle diameters for the samples NT-600 and NT-650 are quite small and are around 20–25 Ω for both. The semi-circle diameter of NT-700 is much higher and can be estimated to be about 2500 Ω. This is likely to be correlated with the structural damage in the nanotube films at higher annealing temperatures (700°C), resulting in the sharply lower electronic conductivity of the sample. This is consistent with a much lower value of capacitance that can be calculated from the CV and charge-discharge curves of the sample NT-700. We conclude that the sample NT-700 has overall an inferior electrochemical performance to the other two samples and focus on the performance of NT-600 and NT-650 in the remainder of the study.
The plots of capacitance as a function of current density for the three samples are shown in Figure 3l. The capacitance of NT-600 drops to about 1015 μF/cm2 at a high current density of 10 μA/cm2. A 30% drop in capacitance of NT-650 is observed at the same high current density for the sample NT-650. Surprisingly, the capacitance retention in the NT-700 was probably the best despite the somewhat compromised structural integrity of this sample. The measured capacitance was, however, significantly lower than that of NT-600 and NT-650.
The measured electrochemical capacitances are generally in line with the values observed in the literature (1–20 mF/cm2).14–28 Our values are close to the bottom of the range. The higher values of capacitance can be obtained when the samples are subjected to additional treatments such as annealing in Ar atmosphere,14,21–23 thermal treatment in H2 atmosphere,15,19 annealing in NH3 atmosphere,16 cathodic biasing of TiO2 in an ethylene glycol electrolyte,18 second anodization with post-annealing in vacuum,20 electrochemical hydrogenation or plasma-treatment,24,25 and cathodic polarization treatment.27,28 Optimization of the electrochemical performance is, however, beyond the scope of this paper. Instead, we focus in the rest of the manuscript on biocompatibility of the TiO2 nanotube films and the possibility of their operation in electrolytes based on physiological liquids.
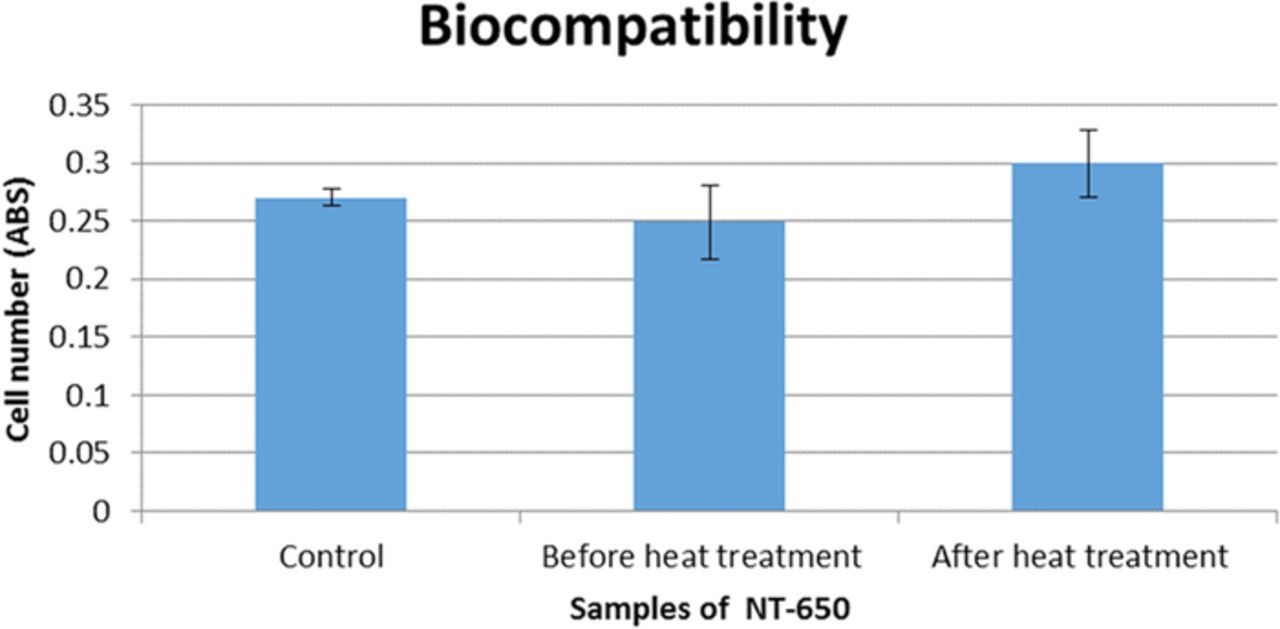
A biocompatibility assessment was carried out using an in-vitro cell culture for the sample of TiO2 nanotubes before and after heat-treatment in Ar at 650°C while the empty well with cells was used as the control group. The cell numbers after cell culturing for 7 days are shown in Figure 4. It can be seen that the cell numbers on the sample before the heat-treatment was similar to that of the control. The cell numbers on the sample after the heat-treatment was higher than that of the control. It indicates that TiO2 nanotubes before and after annealing are biocompatible and the heat-treatment increases the biocompatibility of the sample.
Figure 4. Biocompatibility of NT-650: cell numbers after the contact with a TiO2 nanotube film without annealing in Ar and the sample NT-650 obtained after annealing at 650°C. Cell numbers for the control experiment are also shown. All cell numbers were measured after 7 days of cell culturing.
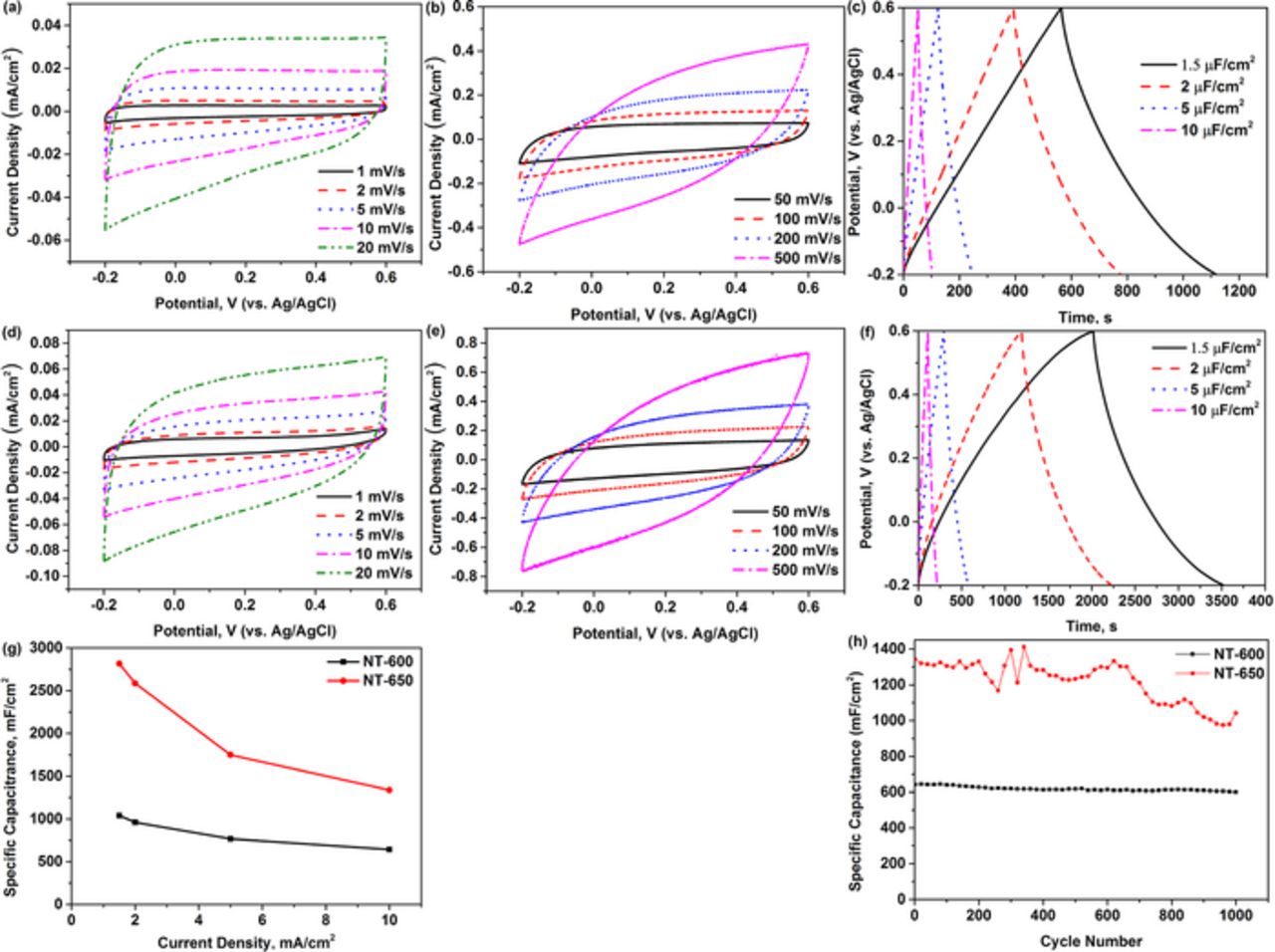
Figure 5 shows the electrochemical performance of NT-600 and NT-650 electrodes in 0.01 M PBS solution. The nanotube electrodes are characterized by CV and galvanostatic charge-discharge. The CV curves of NT-600 and NT-650 are close to the ideal, rectangular shapes at low scan rates (Figure 5a, 5d). Elliptical shape appears in the CV curves at very high scan rates (500 mV/s), indicating that the limitations of the transport phenomena are more pronounced in the three-electrode cell with 0.01 M PBS electrolyte in respect to the cell with the conventional 1 M KCl aqueous electrolytes. It can be concluded from the CV measurement that anodized titanium dioxide nanotubes after annealing are capable of displaying capacitive properties in the physiological electrolytes.
Figure 5. Electrochemical properties of NT-600 and NT-650 in 0.01 M PBS solution: (a, b and c) cyclic voltammetry and galvanostatic charge-discharge curves of NT-600; (d,e and f) cyclic voltammetry and galvanostatic charge-discharge curves of NT-650; (g) the plot of capacitance as a function of current density for the two samples; (h) the cyclic stabilities of the two samples.
Charge-discharge curves confirm these findings, showing approximately triangular curves (Figure 5c, 5f). The measured capacitance of NT-600 is 1040 μF/cm2 at a low current density (1.5 μA/cm2) and over 60% of capacitance can be preserved high current density (10 μA/cm2). Interestingly, the capacitance of NT-650 can be up to 2817 μF/cm2, which is much higher than that of NT-600. We can assume that the mixture of rutile and anatase phases is capable to have higher capacitance than the single anatase phase in the PBS solution. However, this type of material cannot be stable during long cycling as shown in Figure 5h. Indeed, the NT-600 sample has a noticeably better stability after 1000 cycles although its capacitance is limited to only 640 μF/cm2 at a current density of 10 μA/cm2. In summary, it has been confirmed that the TiO2 nanotubes demonstrate biocompatible behavior while, at the same time, being capable of operating in a physiological liquid. We expect that these results will promote further research in this area.
Conclusions
The films of titanium dioxide nanotubes were produced by anodization and were annealed at various temperatures (600, 650 and 700°C). The XRD results indicate that the produced films of TiO2 nanotubes contain anatase and rutile phases. The contribution of the anatase phase decreases with the increase of the annealing temperature, and the rutile phase is dominant in the material annealed at 700°C. The SEM analysis confirms the morphology of nanotubes and indicates changes at higher annealing temperatures, where the structural integrity of the nanotubes declines and nanotubes tend to aggregate into bundles. In line with previous reports, the nanotube films possess capacitive properties, but the capacitance drops noticeably at a higher annealing temperature (700°C).
To conduct preliminary evaluation of TiO2 nanotube electrodes for possible applications in implantable and wearable supercapacitors, a biocompatibility test and electrochemical characterization in a physiological liquid (0.01 M PBS solution) were conducted. It is shown that the nanotube films show biocompatible behavior, with the number of cells in contact with the material increasing over time. Nearly ideal capacitive behavior of TiO2 nanotube films can be demonstrated in a physiological liquid. The specific capacitance of 1040 μF/cm2 is recorded for the nanotube film annealed at 600°C while the sample is also capable of demonstrating a stable cyclic behavior.
Acknowledgments
Financial support from the Australian Research Council under the Discovery Project (DP) Scheme is acknowledged. Authors also acknowledge the use of electron microscopy facilities in the Victorian Node of the Australian National Fabrication Facility (ANFF).