-
PDF
- Split View
-
Views
-
Cite
Cite
Mark I. Stevens, Penelope Greenslade, Ian D. Hogg, Paul Sunnucks, Southern Hemisphere Springtails: Could Any Have Survived Glaciation of Antarctica?, Molecular Biology and Evolution, Volume 23, Issue 5, May 2006, Pages 874–882, https://doi.org/10.1093/molbev/msj073
Close - Share Icon Share
Abstract
Throughout the Southern Hemisphere many terrestrial taxa have circum-Antarctic distributions. This pattern is generally attributed to ongoing dispersal (by wind, water, or migrating birds) or relict Gondwanan distributions. Few of these terrestrial taxa have extant representatives in Antarctica, but such taxa would contribute to our understanding of the evolutionary origins of the continental Antarctic fauna. Either these taxa have survived the harsh climate cooling in Antarctica over the last 23 Myr (Gondwanan/vicariance origin) or they have dispersed there more recently (<2 MYA). In this context, we examined mtDNA (COI) sequence variation in Cryptopygus and related extant Antarctic and subantarctic terrestrial springtails (Collembola). Sequence divergence was estimated under a maximum likelihood model (general time reversible + I + Γ) between individuals from subantarctic islands, Australia, New Zealand, Patagonia, Antarctic Peninsula, and continental Antarctica. Recent dispersal/colonization (<2 MYA) of Cryptopygus species was inferred between some subantarctic islands, and there was a close association between estimated times of divergences based on a molecular clock and proposed geological ages of islands. Most lineages generally grouped according to geographic proximity or by inferred dispersal/colonization pathways. In contrast, the deep divergences found for the four endemic Antarctic species indicate that they represent a continuous chain of descent dating from the break up of Gondwana to the present. We suggest that the diversification of these springtail species (21–11 MYA) in ice-free glacial refugia throughout the Trans-Antarctic Mountains was caused by the glaciation of the Antarctic continent during the middle to late Miocene.
Introduction
Continental landscapes of the Antarctic currently experience extremely low temperatures and limited water availability (Doran et al. 2002). However, paleological evidence indicates that Nothofagus-herb-moss tundra vegetation was present during more moderate climates and persisted in the Trans-Antarctic Mountains of East Antarctica until possibly the late Miocene (∼12–7 MYA) or Pliocene (∼7–2 MYA) (Ashworth and Kuschel 2003; Ashworth and Preece 2003). The fossil record also suggests that some elements of this biota, such as the terrestrial (e.g., weevils and flies) and limnetic faunas (e.g., lymnaeid gastropods, the bivalve Pisidium and at least one species of fish) persisted throughout the Trans-Antarctic Mountains until the late Miocene or Pliocene (Ashworth and Kuschel 2003; Ashworth and Preece 2003; Ashworth and Thompson 2003). It is not clear how much, if any, of this terrestrial Gondwanan fauna survived the glaciation of Antarctica through to the present.
The initial glaciation of Antarctica resulted in long-term habitat fragmentation, and this has been followed by more than 10 major glacial cycles over the last 1 Myr. Only 0.3% of the 14 million km2 of the continent is ice free today (British Antarctic Survey 2004), with a large proportion found in the Trans-Antarctic Mountains where at least some habitat has always been ice free (Hays, Imbrie, and Shackleton 1976; Lawver and Gahagan 2003; Roberts et al. 2003). Accordingly, the extant endemic terrestrial invertebrate fauna of the Antarctic continent is impoverished in terms of species diversity, consisting of a few invertebrates, particularly springtails and mites (Greenslade 1995; Pugh 1997; Hogg and Stevens 2002; Stevens and Hogg 2003, 2006), nematodes (Courtright et al. 2000; Wharton 2003), and tardigrades (McInnes and Pugh 1998). This endemic terrestrial fauna might have survived glaciation of Antarctica in ice-free refuges throughout the Trans-Antarctic Mountains and may be a remnant of a once-abundant and widespread fauna of the Gondwanan supercontinent. Alternatively, the current fauna could have recolonized the Trans-Antarctic Mountains since the last glaciation in a similar manner as postulated in Northern Hemisphere models of postglacial recolonization (e.g., Hewitt 2000).
Although faunal patterns that arose from the sequential break up of Gondwana have been examined for a number of taxa (e.g., Sanmartin and Ronquist 2004; de Queiroz 2005), the invertebrate fauna from the largest fragment of Gondwana, the Antarctic continent, has been little studied. The final break up of Gondwana provided the primary mechanism for climate cooling leading to the glaciation of Antarctica. This is likely to have occurred once the South Tasman Rise had cleared the Oates Land coast of East Antarctica (∼32 MYA) and Drake's passage had opened to deep water circulation (∼28 MYA) (McLoughlin 2001; Lawver and Gahagan 2003). Although the circum-Antarctic currents (and polar front, see fig. 1) were sufficient to isolate Antarctica from other continental landmasses, glaciation of Antarctica did not reach its maximum extent until around 10 MYA (Miller and Mabin 1998; Roberts et al. 2003), and some species may have survived by shifting in a habitat following climate changes in a similar manner to glacier foreground recolonization (e.g., Kaufmann 2001).
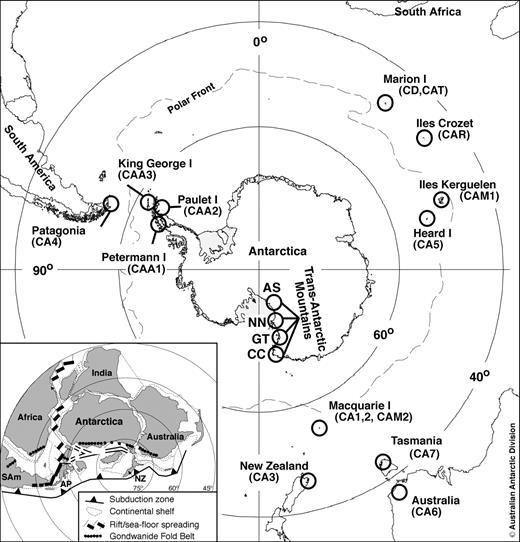
Map of the Southern Hemisphere showing the locations of all samples used. Further details for each site are provided in table 1. Inset (modified from McLoughlin 2001): approximate alignment of the Gondwanan continental landmasses (NZ = New Zealand; AP = Antarctic Peninsula; SAm = South America) that have formed the basis for vicariant hypotheses.
A study examining the origins of the endemic Antarctic fauna in the context of circum-Antarctic fauna may provide further understanding of the evolutionary basis for species' distributions and genetic diversity in the Southern Hemisphere. The genus Cryptopygus (Collembola, Isotomidae) is an ideal model taxon because it is relatively speciose and one of the few taxa that includes extant continental Antarctic species (Wise 1967; Deharveng 1981; Greenslade 1995; Potapov 2001; Deharveng, Potapov, and Bedos 2005; Stevens and Hogg 2006). Here, we used the mtDNA (COI) gene to examine the diversity and relationships of subantarctic and Antarctic springtails throughout the higher latitudes of the Southern Hemisphere (>35°S). Specifically, we tested to what extent the sequence divergence between taxa from the Trans-Antarctic Mountains compared to their circum-Antarctic counterparts would correspond to (1) recolonization—during the Pleistocene/Holocene glacial oscillation (<2 MYA) or (2) persistence—since the gradual glaciation of the Antarctic continent (23–10 MYA) or the break up of Gondwana (>32 MYA).
Materials and Methods
Collections were made between 2001 and 2005 from four continental Antarctic sites (Trans-Antarctic Mountains, Ross Sea Region); in addition, taxonomically related species were targeted from three islands in close proximity to the Antarctic Peninsula, six subantarctic islands, Tasmania and mainland Australia, New Zealand, and Patagonia (fig. 1 and table 1). Springtails were extracted from vegetation, soil, and the underside of rocks using a Tullgren funnel, floatation technique, or aspirator and preserved in 95% ethanol and stored at −20°C. All outgroup individuals were identified by Arne Fjellberg and ingroup individuals by Penelope Greenslade, with vouchers deposited at the South Australian Museum, Adelaide.
Species Examined (with codes), Location (see also fig. 1), and GenBank Accession Numbers
Code . | Species . | Location . | Accession Numbera . |
|---|---|---|---|
| CAA1 | Cryptopygus antarcticus antarcticusWillem, 1901 | Petermann Island | DQ285353–DQ285357 |
| CAA2 | Cryptopygus antarcticus antarcticusWillem, 1901 | Paulet Island | DQ285358 |
| CAA3 | Cryptopygus antarcticus antarcticusWillem, 1901 | King George Island (Potter Cove), South Shetland Islands | DQ285359–DQ285366 |
| CA1 | Cryptopygus sp. 1b | Macquarie Island (Buckles Bay) | DQ285367–DQ285369 |
| CA2 | Cryptopygus sp. 4b | Macquarie Island (Mt Power) | DQ285372 |
| CA3 | Cryptopygus sp. 2b | New Zealand (Fiordland) | DQ285370 |
| CA4 | Cryptopygus sp. 3b | Patagonia (Cerro Bandera) | DQ285371 |
| CA5 | Cryptopygus sp. 4b | Heard Island (Spit Point) | DQ285373–DQ285376 |
| CA6 | Cryptopygus sp. 5b | Australia (East Gippsland) | DQ285377 |
| CA7 | Cryptopygus sp. 6b | Tasmania | DQ285378–DQ285380 |
| CAR | Cryptopygus antarcticus reagensDeharveng, 1981c | Îles Crozet (Île de la Possession) | DQ285386–DQ285389 |
| CAM1 | Cryptopygus antarcticus maximusDeharveng, 1981c | Îles Kerguelen | DQ285381–DQ285384 |
| CAM2 | Cryptopygus antarcticus maximusDeharveng, 1981c | Macquarie Island (Pyramid Lake) | DQ285385 |
| CAT | Cryptopygus antarcticus traveiDeharveng, 1981c | Marion Island (Stony Ridge) | DQ285390 |
| CD | Cryptopygus dubiusDeharveng, 1981 | Marion Island (Stony Ridge) | DQ285395–DQ285398 |
| CC | Cryptopygus cisantarcticusWise, 1967 | Cape Hallett, Trans-Antarctic Mountains, and Antarctica | DQ285391–DQ285394 |
| NN | Neocryptopygus nivicolus Salmon, 1965 | Granite Harbour, Trans-Antarctic Mountains, and Antarctica | DQ285403–DQ285404 |
| GT | Gressittacantha terranovaWise, 1967 | Terra Nova Bay, Trans-Antarctic Mountains, Antarctica | DQ285399–DQ285402 |
| AS | Antarctophorus subpolaris (Salmon, 1962) | Beardmore Glacier, Trans-Antarctic Mountains, and Antarctica | DQ285405–DQ285406 |
| Outgroup taxa | Pseudisotoma sensibilis (Tullberg, 1871) | Greenland (Maniitsoq) | DQ285296d |
| Isotoma anglicana Lubbock, 1862 | Canadian arctic (Cornwallis Island) | AY383534e | |
| Vertagopus brevicaudus (Carpenter, 1900) | Canadian arctic (Cornwallis Island) | AY665354e | |
| Desoria klovstadi (Carpenter, 1902)f | Antarctica, Trans-Antarctic Mountains (Cape Hallett) | DQ285297d | |
| Archisotoma polaris Fjellberg & Pouinsot, 1995 | Norway (Svalbard) | DQ285298d |
Code . | Species . | Location . | Accession Numbera . |
|---|---|---|---|
| CAA1 | Cryptopygus antarcticus antarcticusWillem, 1901 | Petermann Island | DQ285353–DQ285357 |
| CAA2 | Cryptopygus antarcticus antarcticusWillem, 1901 | Paulet Island | DQ285358 |
| CAA3 | Cryptopygus antarcticus antarcticusWillem, 1901 | King George Island (Potter Cove), South Shetland Islands | DQ285359–DQ285366 |
| CA1 | Cryptopygus sp. 1b | Macquarie Island (Buckles Bay) | DQ285367–DQ285369 |
| CA2 | Cryptopygus sp. 4b | Macquarie Island (Mt Power) | DQ285372 |
| CA3 | Cryptopygus sp. 2b | New Zealand (Fiordland) | DQ285370 |
| CA4 | Cryptopygus sp. 3b | Patagonia (Cerro Bandera) | DQ285371 |
| CA5 | Cryptopygus sp. 4b | Heard Island (Spit Point) | DQ285373–DQ285376 |
| CA6 | Cryptopygus sp. 5b | Australia (East Gippsland) | DQ285377 |
| CA7 | Cryptopygus sp. 6b | Tasmania | DQ285378–DQ285380 |
| CAR | Cryptopygus antarcticus reagensDeharveng, 1981c | Îles Crozet (Île de la Possession) | DQ285386–DQ285389 |
| CAM1 | Cryptopygus antarcticus maximusDeharveng, 1981c | Îles Kerguelen | DQ285381–DQ285384 |
| CAM2 | Cryptopygus antarcticus maximusDeharveng, 1981c | Macquarie Island (Pyramid Lake) | DQ285385 |
| CAT | Cryptopygus antarcticus traveiDeharveng, 1981c | Marion Island (Stony Ridge) | DQ285390 |
| CD | Cryptopygus dubiusDeharveng, 1981 | Marion Island (Stony Ridge) | DQ285395–DQ285398 |
| CC | Cryptopygus cisantarcticusWise, 1967 | Cape Hallett, Trans-Antarctic Mountains, and Antarctica | DQ285391–DQ285394 |
| NN | Neocryptopygus nivicolus Salmon, 1965 | Granite Harbour, Trans-Antarctic Mountains, and Antarctica | DQ285403–DQ285404 |
| GT | Gressittacantha terranovaWise, 1967 | Terra Nova Bay, Trans-Antarctic Mountains, Antarctica | DQ285399–DQ285402 |
| AS | Antarctophorus subpolaris (Salmon, 1962) | Beardmore Glacier, Trans-Antarctic Mountains, and Antarctica | DQ285405–DQ285406 |
| Outgroup taxa | Pseudisotoma sensibilis (Tullberg, 1871) | Greenland (Maniitsoq) | DQ285296d |
| Isotoma anglicana Lubbock, 1862 | Canadian arctic (Cornwallis Island) | AY383534e | |
| Vertagopus brevicaudus (Carpenter, 1900) | Canadian arctic (Cornwallis Island) | AY665354e | |
| Desoria klovstadi (Carpenter, 1902)f | Antarctica, Trans-Antarctic Mountains (Cape Hallett) | DQ285297d | |
| Archisotoma polaris Fjellberg & Pouinsot, 1995 | Norway (Svalbard) | DQ285298d |
GenBank (National Center for Biotechnology Information [NCBI]) accession numbers for new data obtained from this study (unique haplotypes only).
Taxa suggested as species, but not formerly described by Deharveng (1981) or from the present study.
Subspecies (formally described by Deharveng 1981) suggested as species based on molecular data in the present study.
GenBank (NCBI) accession numbers for new data obtained from Stevens et al. (2006).
GenBank (NCBI) accession numbers for new data obtained from Hogg and Hebert (2004).
Recently redescribed (formally Isotoma klovstadi) by Stevens et al. (2006).
Species Examined (with codes), Location (see also fig. 1), and GenBank Accession Numbers
Code . | Species . | Location . | Accession Numbera . |
|---|---|---|---|
| CAA1 | Cryptopygus antarcticus antarcticusWillem, 1901 | Petermann Island | DQ285353–DQ285357 |
| CAA2 | Cryptopygus antarcticus antarcticusWillem, 1901 | Paulet Island | DQ285358 |
| CAA3 | Cryptopygus antarcticus antarcticusWillem, 1901 | King George Island (Potter Cove), South Shetland Islands | DQ285359–DQ285366 |
| CA1 | Cryptopygus sp. 1b | Macquarie Island (Buckles Bay) | DQ285367–DQ285369 |
| CA2 | Cryptopygus sp. 4b | Macquarie Island (Mt Power) | DQ285372 |
| CA3 | Cryptopygus sp. 2b | New Zealand (Fiordland) | DQ285370 |
| CA4 | Cryptopygus sp. 3b | Patagonia (Cerro Bandera) | DQ285371 |
| CA5 | Cryptopygus sp. 4b | Heard Island (Spit Point) | DQ285373–DQ285376 |
| CA6 | Cryptopygus sp. 5b | Australia (East Gippsland) | DQ285377 |
| CA7 | Cryptopygus sp. 6b | Tasmania | DQ285378–DQ285380 |
| CAR | Cryptopygus antarcticus reagensDeharveng, 1981c | Îles Crozet (Île de la Possession) | DQ285386–DQ285389 |
| CAM1 | Cryptopygus antarcticus maximusDeharveng, 1981c | Îles Kerguelen | DQ285381–DQ285384 |
| CAM2 | Cryptopygus antarcticus maximusDeharveng, 1981c | Macquarie Island (Pyramid Lake) | DQ285385 |
| CAT | Cryptopygus antarcticus traveiDeharveng, 1981c | Marion Island (Stony Ridge) | DQ285390 |
| CD | Cryptopygus dubiusDeharveng, 1981 | Marion Island (Stony Ridge) | DQ285395–DQ285398 |
| CC | Cryptopygus cisantarcticusWise, 1967 | Cape Hallett, Trans-Antarctic Mountains, and Antarctica | DQ285391–DQ285394 |
| NN | Neocryptopygus nivicolus Salmon, 1965 | Granite Harbour, Trans-Antarctic Mountains, and Antarctica | DQ285403–DQ285404 |
| GT | Gressittacantha terranovaWise, 1967 | Terra Nova Bay, Trans-Antarctic Mountains, Antarctica | DQ285399–DQ285402 |
| AS | Antarctophorus subpolaris (Salmon, 1962) | Beardmore Glacier, Trans-Antarctic Mountains, and Antarctica | DQ285405–DQ285406 |
| Outgroup taxa | Pseudisotoma sensibilis (Tullberg, 1871) | Greenland (Maniitsoq) | DQ285296d |
| Isotoma anglicana Lubbock, 1862 | Canadian arctic (Cornwallis Island) | AY383534e | |
| Vertagopus brevicaudus (Carpenter, 1900) | Canadian arctic (Cornwallis Island) | AY665354e | |
| Desoria klovstadi (Carpenter, 1902)f | Antarctica, Trans-Antarctic Mountains (Cape Hallett) | DQ285297d | |
| Archisotoma polaris Fjellberg & Pouinsot, 1995 | Norway (Svalbard) | DQ285298d |
Code . | Species . | Location . | Accession Numbera . |
|---|---|---|---|
| CAA1 | Cryptopygus antarcticus antarcticusWillem, 1901 | Petermann Island | DQ285353–DQ285357 |
| CAA2 | Cryptopygus antarcticus antarcticusWillem, 1901 | Paulet Island | DQ285358 |
| CAA3 | Cryptopygus antarcticus antarcticusWillem, 1901 | King George Island (Potter Cove), South Shetland Islands | DQ285359–DQ285366 |
| CA1 | Cryptopygus sp. 1b | Macquarie Island (Buckles Bay) | DQ285367–DQ285369 |
| CA2 | Cryptopygus sp. 4b | Macquarie Island (Mt Power) | DQ285372 |
| CA3 | Cryptopygus sp. 2b | New Zealand (Fiordland) | DQ285370 |
| CA4 | Cryptopygus sp. 3b | Patagonia (Cerro Bandera) | DQ285371 |
| CA5 | Cryptopygus sp. 4b | Heard Island (Spit Point) | DQ285373–DQ285376 |
| CA6 | Cryptopygus sp. 5b | Australia (East Gippsland) | DQ285377 |
| CA7 | Cryptopygus sp. 6b | Tasmania | DQ285378–DQ285380 |
| CAR | Cryptopygus antarcticus reagensDeharveng, 1981c | Îles Crozet (Île de la Possession) | DQ285386–DQ285389 |
| CAM1 | Cryptopygus antarcticus maximusDeharveng, 1981c | Îles Kerguelen | DQ285381–DQ285384 |
| CAM2 | Cryptopygus antarcticus maximusDeharveng, 1981c | Macquarie Island (Pyramid Lake) | DQ285385 |
| CAT | Cryptopygus antarcticus traveiDeharveng, 1981c | Marion Island (Stony Ridge) | DQ285390 |
| CD | Cryptopygus dubiusDeharveng, 1981 | Marion Island (Stony Ridge) | DQ285395–DQ285398 |
| CC | Cryptopygus cisantarcticusWise, 1967 | Cape Hallett, Trans-Antarctic Mountains, and Antarctica | DQ285391–DQ285394 |
| NN | Neocryptopygus nivicolus Salmon, 1965 | Granite Harbour, Trans-Antarctic Mountains, and Antarctica | DQ285403–DQ285404 |
| GT | Gressittacantha terranovaWise, 1967 | Terra Nova Bay, Trans-Antarctic Mountains, Antarctica | DQ285399–DQ285402 |
| AS | Antarctophorus subpolaris (Salmon, 1962) | Beardmore Glacier, Trans-Antarctic Mountains, and Antarctica | DQ285405–DQ285406 |
| Outgroup taxa | Pseudisotoma sensibilis (Tullberg, 1871) | Greenland (Maniitsoq) | DQ285296d |
| Isotoma anglicana Lubbock, 1862 | Canadian arctic (Cornwallis Island) | AY383534e | |
| Vertagopus brevicaudus (Carpenter, 1900) | Canadian arctic (Cornwallis Island) | AY665354e | |
| Desoria klovstadi (Carpenter, 1902)f | Antarctica, Trans-Antarctic Mountains (Cape Hallett) | DQ285297d | |
| Archisotoma polaris Fjellberg & Pouinsot, 1995 | Norway (Svalbard) | DQ285298d |
GenBank (National Center for Biotechnology Information [NCBI]) accession numbers for new data obtained from this study (unique haplotypes only).
Taxa suggested as species, but not formerly described by Deharveng (1981) or from the present study.
Subspecies (formally described by Deharveng 1981) suggested as species based on molecular data in the present study.
GenBank (NCBI) accession numbers for new data obtained from Stevens et al. (2006).
GenBank (NCBI) accession numbers for new data obtained from Hogg and Hebert (2004).
Recently redescribed (formally Isotoma klovstadi) by Stevens et al. (2006).
Total genomic DNA was extracted from entire individuals using a “salting out” technique (Sunnucks and Hales 1996). Polymerase chain reaction (PCR) amplification was carried out using a 10-μl reaction volume with the primers LCO1490 and HCO2198 (Folmer et al. 1994) to target a fragment of the COI gene; further details for PCR are in Stevens and Hogg (2003). All reaction products were purified with SAPEXO (USB Corp., Cleveland, Ohio). Purified PCR products were sequenced (using the forward and reverse primers) directly with BigDye Terminator chemistry (Applied Biosystems, Foster City, Calif.). Sequencing was performed on a capillary ABI3730 genetic analyzer (Applied Biosystems) at the Allan Wilson Centre Genome Service, Massey University or on a MegaBACE DNA Analysis System (Amersham Biosciences, Buckinghamshire, U.K.) at the University of Waikato DNA sequencing facility.
Sequences were checked for consistency with springtail DNA using the GenBank BlastN search. In addition, sequences were checked for open reading frames (using MacClade ver. 4.05; D. R. Maddison and W. P. Maddison 2000) because failure to confirm these may indicate the presence of nuclear copies or other unintended sequence types (Sunnucks and Hales 1996). We used five outgroup taxa from several locations, including continental Antarctica (table 1). Sequences were aligned in SEQUENCHER (Gene Codes ver. 4.5) sequence editor and analyzed using PAUP* 4.0b10 (Swofford 2002). χ2 tests (implemented in PAUP*) were used to test the hypothesis of homogeneity of base frequencies among sequences; we were unable to reject the hypothesis for all sites (
Saturation and base composition heterogeneity were dealt with in the ML analyses by recoding the third codon position as purines (R) and pyrimidines (Y) (Delsuc, Phillips, and Penny 2003; Phillips and Penny 2003), while the first and second positions were kept as nucleotides (hereafter referred to as RY-coded data; alignment available as Supplementary Material online). Modeltest ver. 3.7 (Posada and Crandall 1998) was then used to determine the appropriate model parameters for ML heuristic searches, in which the GTR + I + Γ (−ln L = 3763.52; rate matrix: A–C = 0.3407, A–G = 0.9603, A–T = 0.9838, C–G = 0.1890, C–T = 3.6628, G–T = 1.0000; I = 0.4314, Γ = 0.2926; with base frequencies set to A = 0.2265, C = 0.2142, G = 0.2381, T = 0.3213) was found to be the best fit to the RY-coded data; all other options in PAUP* remained as default for the ML analyses. ML bootstrap analyses were conducted with 500 replicates (Felsenstein 1985). Comparisons of log-likelihood scores (using χ2 tests) for trees with and without a molecular clock enforced (performed on the original nucleotide alignment) indicated that these sequences were evolving in a clocklike manner. Subsequently, we estimated age among lineages based on an ML model (Arbogast et al. 2002; Ho et al. 2005; see table 2) using an arthropod strict molecular clock conservative calibration of 1.5%–2.3% divergence per million year derived from comparisons between geological and molecular data (Brower 1994; Gaunt and Miles 2002; Quek et al. 2004). This range of molecular rates has been applied to Collembola with outcomes that fit paleoclimatic assumptions (Garrick et al. 2004).
Species . | Code . | Within Variabilityc . | CAA1 . | CAA2 . | CAA3 . | CA1 . | CA2 . | CA3 . | CA4 . | CA5 . | CA6 . | CA7 . | CAM1 . | CAM2 . | CAR . | CAT . | CD . | CC . | GT . | NN . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryptopygus antarcticus antarcticus | CAA1 | 0.002–0.038 (5) | ||||||||||||||||||
| CAA2 | 0.000–0.000 (1) | 0.040–0.054 | ||||||||||||||||||
| CAA3 | 0.002–0.026 (8) | 0.018–0.063 | 0.044–0.063 | |||||||||||||||||
| “Cryptopygus antarcticus” group | ||||||||||||||||||||
| Cryptopygus sp. 1, Macquarie I. | CA1 | 0.002–0.014 (3) | 0.294–0.331 | 0.343–0.361 | 0.276–0.317 | |||||||||||||||
| Cryptopygus sp. 4, Macquarie I. | CA2 | 0.000–0.000 (1) | 0.417–0.456 | 0.436 | 0.389–0.433 | 0.480–0.496 | ||||||||||||||
| Cryptopygus sp. 2, New Zealand | CA3 | 0.000–0.000 (1) | 0.478–0.552 | 0.527 | 0.445–0.460 | 0.374–0.384 | 0.331 | |||||||||||||
| Cryptopygus sp. 3, Patagonia | CA4 | 0.000–0.000 (1) | 0.355–0.388 | 0.355 | 0.360–0.381 | 0.282–0.295 | 0.292 | 0.237 | ||||||||||||
| Cryptopygus sp. 4, Heard I. | CA5 | 0.002–0.007 (4) | 0.424–0.483 | 0.443–0.457 | 0.394–0.454 | 0.492–0.524 | 0.007–0.011 | 0.323–0.340 | 0.299–0.309 | |||||||||||
| Cryptopygus sp. 5, Australia | CA6 | 0.000–0.000 (1) | 0.384–0.408 | 0.413 | 0.346–0.389 | 0.299–0.307 | 0.391 | 0.296 | 0.260 | 0.402–0.414 | ||||||||||
| Cryptopygus sp. 6, Tasmania | CA7 | 0.002–0.005 (3) | 0.425–0.471 | 0.474–0.479 | 0.403–0.443 | 0.407–0.423 | 0.391–0.399 | 0.385–0.394 | 0.320–0.328 | 0.390–0.411 | 0.259–0.261 | |||||||||
| Cryptopygus antarcticus maximus (Is. Kerguelen) | CAM1 | 0.002–0.012 (4) | 0.140–0.167 | 0.167–0.173 | 0.123–0.160 | 0.322–0.386 | 0.485–0.517 | 0.438–0.476 | 0.362–0.373 | 0.492–0.542 | 0.408–0.430 | 0.401–0.441 | ||||||||
| C. antarcticus maximus (Macquarie I.) | CAM2 | 0.000–0.000 (1) | 0.141–0.165 | 0.174 | 0.134–0.157 | 0.334–0.363 | 0.468 | 0.470 | 0.380 | 0.475–0.491 | 0.406 | 0.413–0.420 | 0.022–0.032 | |||||||
| Cryptopygus antarcticus reagens (I. Crozet) | CAR | 0.002–0.009 (4) | 0.383–0.442 | 0.380–0.402 | 0.393–0.470 | 0.415–0.446 | 0.449–0.479 | 0.425–0.435 | 0.365–0.390 | 0.453–0.499 | 0.316–0.338 | 0.393–0.427 | 0.352–0.405 | 0.378–0.390 | ||||||
| Cryptopygus antarcticus travei (Marion I.) | CAT | 0.000–0.000 (1) | 0.327–0.367 | 0.358 | 0.303–0.351 | 0.315–0.326 | 0.408 | 0.422 | 0.402 | 0.419–0.432 | 0.282 | 0.425–0.429 | 0.389–0.412 | 0.381 | 0.260–0.275 | |||||
| Cryptopygus dubius (Marion I.) | CD | 0.003–0.009 (4) | 0.355–0.403 | 0.378–0.386 | 0.327–0.351 | 0.351–0.379 | 0.395–0.411 | 0.440–0.456 | 0.331–0.339 | 0.406–0.435 | 0.360–0.365 | 0.397–0.383 | 0.406–0.454 | 0.387–0.389 | 0.371–0.415 | 0.324–0.341 | ||||
| Trans-Antarctic Mountains | ||||||||||||||||||||
| Cryptopygus cisantarcticus | CC | 0.009–0.022 (4) | 0.367–0.424 | 0.370–0.391 | 0.357–0.439 | 0.303–0.367 | 0.326–0.369 | 0.289–0.329 | 0.255–0.277 | 0.335–0.391 | 0.279–0.317 | 0.378–0.429 | 0.369–0.419 | 0.355–0.400 | 0.334–0.378 | 0.333–0.374 | 0.333–0.383 | |||
| Gressittacantha terranova | GT | 0.003–0.039 (4) | 0.365–0.392 | 0.403–0.428 | 0.368–0.426 | 0.284–0.301 | 0.398–0.423 | 0.298–0.329 | 0.272–0.288 | 0.397–0.448 | 0.267–0.293 | 0.310–0.345 | 0.393–0.438 | 0.396–0.421 | 0.331–0.404 | 0.372–0.392 | 0.353–0.387 | 0.317–0.360 | ||
| Neocryptopygus nivicolus | NN | 0.002 (2) | 0.357–0.422 | 0.377–0.381 | 0.353–0.370 | 0.376–0.385 | 0.339–0.342 | 0.362–0.366 | 0.318–0.322 | 0.345–0.360 | 0.315–0.319 | 0.336–0.347 | 0.346–0.378 | 0.373–0.377 | 0.409–0.441 | 0.389–0.393 | 0.410–0.418 | 0.333–0.383 | 0.285–0.319 | |
| Antarctophorus subpolaris | AS | 0.003 (2) | 0.395–0.431 | 0.416–0.424 | 0.369–0.400 | 0.372–0.363 | 0.415 | 0.416 | 0.366 | 0.426–0.440 | 0.339–0.349 | 0.367–0.382 | 0.437–0.471 | 0.462 | 0.409–0.444 | 0.314 | 0.347–0.364 | 0.303–0.348 | 0.356–0.390 | 0.309–0.320 |
Species . | Code . | Within Variabilityc . | CAA1 . | CAA2 . | CAA3 . | CA1 . | CA2 . | CA3 . | CA4 . | CA5 . | CA6 . | CA7 . | CAM1 . | CAM2 . | CAR . | CAT . | CD . | CC . | GT . | NN . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryptopygus antarcticus antarcticus | CAA1 | 0.002–0.038 (5) | ||||||||||||||||||
| CAA2 | 0.000–0.000 (1) | 0.040–0.054 | ||||||||||||||||||
| CAA3 | 0.002–0.026 (8) | 0.018–0.063 | 0.044–0.063 | |||||||||||||||||
| “Cryptopygus antarcticus” group | ||||||||||||||||||||
| Cryptopygus sp. 1, Macquarie I. | CA1 | 0.002–0.014 (3) | 0.294–0.331 | 0.343–0.361 | 0.276–0.317 | |||||||||||||||
| Cryptopygus sp. 4, Macquarie I. | CA2 | 0.000–0.000 (1) | 0.417–0.456 | 0.436 | 0.389–0.433 | 0.480–0.496 | ||||||||||||||
| Cryptopygus sp. 2, New Zealand | CA3 | 0.000–0.000 (1) | 0.478–0.552 | 0.527 | 0.445–0.460 | 0.374–0.384 | 0.331 | |||||||||||||
| Cryptopygus sp. 3, Patagonia | CA4 | 0.000–0.000 (1) | 0.355–0.388 | 0.355 | 0.360–0.381 | 0.282–0.295 | 0.292 | 0.237 | ||||||||||||
| Cryptopygus sp. 4, Heard I. | CA5 | 0.002–0.007 (4) | 0.424–0.483 | 0.443–0.457 | 0.394–0.454 | 0.492–0.524 | 0.007–0.011 | 0.323–0.340 | 0.299–0.309 | |||||||||||
| Cryptopygus sp. 5, Australia | CA6 | 0.000–0.000 (1) | 0.384–0.408 | 0.413 | 0.346–0.389 | 0.299–0.307 | 0.391 | 0.296 | 0.260 | 0.402–0.414 | ||||||||||
| Cryptopygus sp. 6, Tasmania | CA7 | 0.002–0.005 (3) | 0.425–0.471 | 0.474–0.479 | 0.403–0.443 | 0.407–0.423 | 0.391–0.399 | 0.385–0.394 | 0.320–0.328 | 0.390–0.411 | 0.259–0.261 | |||||||||
| Cryptopygus antarcticus maximus (Is. Kerguelen) | CAM1 | 0.002–0.012 (4) | 0.140–0.167 | 0.167–0.173 | 0.123–0.160 | 0.322–0.386 | 0.485–0.517 | 0.438–0.476 | 0.362–0.373 | 0.492–0.542 | 0.408–0.430 | 0.401–0.441 | ||||||||
| C. antarcticus maximus (Macquarie I.) | CAM2 | 0.000–0.000 (1) | 0.141–0.165 | 0.174 | 0.134–0.157 | 0.334–0.363 | 0.468 | 0.470 | 0.380 | 0.475–0.491 | 0.406 | 0.413–0.420 | 0.022–0.032 | |||||||
| Cryptopygus antarcticus reagens (I. Crozet) | CAR | 0.002–0.009 (4) | 0.383–0.442 | 0.380–0.402 | 0.393–0.470 | 0.415–0.446 | 0.449–0.479 | 0.425–0.435 | 0.365–0.390 | 0.453–0.499 | 0.316–0.338 | 0.393–0.427 | 0.352–0.405 | 0.378–0.390 | ||||||
| Cryptopygus antarcticus travei (Marion I.) | CAT | 0.000–0.000 (1) | 0.327–0.367 | 0.358 | 0.303–0.351 | 0.315–0.326 | 0.408 | 0.422 | 0.402 | 0.419–0.432 | 0.282 | 0.425–0.429 | 0.389–0.412 | 0.381 | 0.260–0.275 | |||||
| Cryptopygus dubius (Marion I.) | CD | 0.003–0.009 (4) | 0.355–0.403 | 0.378–0.386 | 0.327–0.351 | 0.351–0.379 | 0.395–0.411 | 0.440–0.456 | 0.331–0.339 | 0.406–0.435 | 0.360–0.365 | 0.397–0.383 | 0.406–0.454 | 0.387–0.389 | 0.371–0.415 | 0.324–0.341 | ||||
| Trans-Antarctic Mountains | ||||||||||||||||||||
| Cryptopygus cisantarcticus | CC | 0.009–0.022 (4) | 0.367–0.424 | 0.370–0.391 | 0.357–0.439 | 0.303–0.367 | 0.326–0.369 | 0.289–0.329 | 0.255–0.277 | 0.335–0.391 | 0.279–0.317 | 0.378–0.429 | 0.369–0.419 | 0.355–0.400 | 0.334–0.378 | 0.333–0.374 | 0.333–0.383 | |||
| Gressittacantha terranova | GT | 0.003–0.039 (4) | 0.365–0.392 | 0.403–0.428 | 0.368–0.426 | 0.284–0.301 | 0.398–0.423 | 0.298–0.329 | 0.272–0.288 | 0.397–0.448 | 0.267–0.293 | 0.310–0.345 | 0.393–0.438 | 0.396–0.421 | 0.331–0.404 | 0.372–0.392 | 0.353–0.387 | 0.317–0.360 | ||
| Neocryptopygus nivicolus | NN | 0.002 (2) | 0.357–0.422 | 0.377–0.381 | 0.353–0.370 | 0.376–0.385 | 0.339–0.342 | 0.362–0.366 | 0.318–0.322 | 0.345–0.360 | 0.315–0.319 | 0.336–0.347 | 0.346–0.378 | 0.373–0.377 | 0.409–0.441 | 0.389–0.393 | 0.410–0.418 | 0.333–0.383 | 0.285–0.319 | |
| Antarctophorus subpolaris | AS | 0.003 (2) | 0.395–0.431 | 0.416–0.424 | 0.369–0.400 | 0.372–0.363 | 0.415 | 0.416 | 0.366 | 0.426–0.440 | 0.339–0.349 | 0.367–0.382 | 0.437–0.471 | 0.462 | 0.409–0.444 | 0.314 | 0.347–0.364 | 0.303–0.348 | 0.356–0.390 | 0.309–0.320 |
Rate matrix: A–C = 0.9223, A–G = 5.4335, A–T = 2.8463, C–G = 0.9321, C–T = 8.6194, G–T = 1.0000; I = 0.5069, Γ = 1.1495; base frequencies set to A = 0.2929, C = 0.1899, G = 0.1452, T = 0.3720).
See Supplementary table S1 (Supplementary Material online) for the complete table of sequence divergences (ML and uncorrected) among the ingroup and outgroup taxa).
Number of unique haplotypes (all taxa, all sites) shown in parentheses.
Species . | Code . | Within Variabilityc . | CAA1 . | CAA2 . | CAA3 . | CA1 . | CA2 . | CA3 . | CA4 . | CA5 . | CA6 . | CA7 . | CAM1 . | CAM2 . | CAR . | CAT . | CD . | CC . | GT . | NN . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryptopygus antarcticus antarcticus | CAA1 | 0.002–0.038 (5) | ||||||||||||||||||
| CAA2 | 0.000–0.000 (1) | 0.040–0.054 | ||||||||||||||||||
| CAA3 | 0.002–0.026 (8) | 0.018–0.063 | 0.044–0.063 | |||||||||||||||||
| “Cryptopygus antarcticus” group | ||||||||||||||||||||
| Cryptopygus sp. 1, Macquarie I. | CA1 | 0.002–0.014 (3) | 0.294–0.331 | 0.343–0.361 | 0.276–0.317 | |||||||||||||||
| Cryptopygus sp. 4, Macquarie I. | CA2 | 0.000–0.000 (1) | 0.417–0.456 | 0.436 | 0.389–0.433 | 0.480–0.496 | ||||||||||||||
| Cryptopygus sp. 2, New Zealand | CA3 | 0.000–0.000 (1) | 0.478–0.552 | 0.527 | 0.445–0.460 | 0.374–0.384 | 0.331 | |||||||||||||
| Cryptopygus sp. 3, Patagonia | CA4 | 0.000–0.000 (1) | 0.355–0.388 | 0.355 | 0.360–0.381 | 0.282–0.295 | 0.292 | 0.237 | ||||||||||||
| Cryptopygus sp. 4, Heard I. | CA5 | 0.002–0.007 (4) | 0.424–0.483 | 0.443–0.457 | 0.394–0.454 | 0.492–0.524 | 0.007–0.011 | 0.323–0.340 | 0.299–0.309 | |||||||||||
| Cryptopygus sp. 5, Australia | CA6 | 0.000–0.000 (1) | 0.384–0.408 | 0.413 | 0.346–0.389 | 0.299–0.307 | 0.391 | 0.296 | 0.260 | 0.402–0.414 | ||||||||||
| Cryptopygus sp. 6, Tasmania | CA7 | 0.002–0.005 (3) | 0.425–0.471 | 0.474–0.479 | 0.403–0.443 | 0.407–0.423 | 0.391–0.399 | 0.385–0.394 | 0.320–0.328 | 0.390–0.411 | 0.259–0.261 | |||||||||
| Cryptopygus antarcticus maximus (Is. Kerguelen) | CAM1 | 0.002–0.012 (4) | 0.140–0.167 | 0.167–0.173 | 0.123–0.160 | 0.322–0.386 | 0.485–0.517 | 0.438–0.476 | 0.362–0.373 | 0.492–0.542 | 0.408–0.430 | 0.401–0.441 | ||||||||
| C. antarcticus maximus (Macquarie I.) | CAM2 | 0.000–0.000 (1) | 0.141–0.165 | 0.174 | 0.134–0.157 | 0.334–0.363 | 0.468 | 0.470 | 0.380 | 0.475–0.491 | 0.406 | 0.413–0.420 | 0.022–0.032 | |||||||
| Cryptopygus antarcticus reagens (I. Crozet) | CAR | 0.002–0.009 (4) | 0.383–0.442 | 0.380–0.402 | 0.393–0.470 | 0.415–0.446 | 0.449–0.479 | 0.425–0.435 | 0.365–0.390 | 0.453–0.499 | 0.316–0.338 | 0.393–0.427 | 0.352–0.405 | 0.378–0.390 | ||||||
| Cryptopygus antarcticus travei (Marion I.) | CAT | 0.000–0.000 (1) | 0.327–0.367 | 0.358 | 0.303–0.351 | 0.315–0.326 | 0.408 | 0.422 | 0.402 | 0.419–0.432 | 0.282 | 0.425–0.429 | 0.389–0.412 | 0.381 | 0.260–0.275 | |||||
| Cryptopygus dubius (Marion I.) | CD | 0.003–0.009 (4) | 0.355–0.403 | 0.378–0.386 | 0.327–0.351 | 0.351–0.379 | 0.395–0.411 | 0.440–0.456 | 0.331–0.339 | 0.406–0.435 | 0.360–0.365 | 0.397–0.383 | 0.406–0.454 | 0.387–0.389 | 0.371–0.415 | 0.324–0.341 | ||||
| Trans-Antarctic Mountains | ||||||||||||||||||||
| Cryptopygus cisantarcticus | CC | 0.009–0.022 (4) | 0.367–0.424 | 0.370–0.391 | 0.357–0.439 | 0.303–0.367 | 0.326–0.369 | 0.289–0.329 | 0.255–0.277 | 0.335–0.391 | 0.279–0.317 | 0.378–0.429 | 0.369–0.419 | 0.355–0.400 | 0.334–0.378 | 0.333–0.374 | 0.333–0.383 | |||
| Gressittacantha terranova | GT | 0.003–0.039 (4) | 0.365–0.392 | 0.403–0.428 | 0.368–0.426 | 0.284–0.301 | 0.398–0.423 | 0.298–0.329 | 0.272–0.288 | 0.397–0.448 | 0.267–0.293 | 0.310–0.345 | 0.393–0.438 | 0.396–0.421 | 0.331–0.404 | 0.372–0.392 | 0.353–0.387 | 0.317–0.360 | ||
| Neocryptopygus nivicolus | NN | 0.002 (2) | 0.357–0.422 | 0.377–0.381 | 0.353–0.370 | 0.376–0.385 | 0.339–0.342 | 0.362–0.366 | 0.318–0.322 | 0.345–0.360 | 0.315–0.319 | 0.336–0.347 | 0.346–0.378 | 0.373–0.377 | 0.409–0.441 | 0.389–0.393 | 0.410–0.418 | 0.333–0.383 | 0.285–0.319 | |
| Antarctophorus subpolaris | AS | 0.003 (2) | 0.395–0.431 | 0.416–0.424 | 0.369–0.400 | 0.372–0.363 | 0.415 | 0.416 | 0.366 | 0.426–0.440 | 0.339–0.349 | 0.367–0.382 | 0.437–0.471 | 0.462 | 0.409–0.444 | 0.314 | 0.347–0.364 | 0.303–0.348 | 0.356–0.390 | 0.309–0.320 |
Species . | Code . | Within Variabilityc . | CAA1 . | CAA2 . | CAA3 . | CA1 . | CA2 . | CA3 . | CA4 . | CA5 . | CA6 . | CA7 . | CAM1 . | CAM2 . | CAR . | CAT . | CD . | CC . | GT . | NN . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryptopygus antarcticus antarcticus | CAA1 | 0.002–0.038 (5) | ||||||||||||||||||
| CAA2 | 0.000–0.000 (1) | 0.040–0.054 | ||||||||||||||||||
| CAA3 | 0.002–0.026 (8) | 0.018–0.063 | 0.044–0.063 | |||||||||||||||||
| “Cryptopygus antarcticus” group | ||||||||||||||||||||
| Cryptopygus sp. 1, Macquarie I. | CA1 | 0.002–0.014 (3) | 0.294–0.331 | 0.343–0.361 | 0.276–0.317 | |||||||||||||||
| Cryptopygus sp. 4, Macquarie I. | CA2 | 0.000–0.000 (1) | 0.417–0.456 | 0.436 | 0.389–0.433 | 0.480–0.496 | ||||||||||||||
| Cryptopygus sp. 2, New Zealand | CA3 | 0.000–0.000 (1) | 0.478–0.552 | 0.527 | 0.445–0.460 | 0.374–0.384 | 0.331 | |||||||||||||
| Cryptopygus sp. 3, Patagonia | CA4 | 0.000–0.000 (1) | 0.355–0.388 | 0.355 | 0.360–0.381 | 0.282–0.295 | 0.292 | 0.237 | ||||||||||||
| Cryptopygus sp. 4, Heard I. | CA5 | 0.002–0.007 (4) | 0.424–0.483 | 0.443–0.457 | 0.394–0.454 | 0.492–0.524 | 0.007–0.011 | 0.323–0.340 | 0.299–0.309 | |||||||||||
| Cryptopygus sp. 5, Australia | CA6 | 0.000–0.000 (1) | 0.384–0.408 | 0.413 | 0.346–0.389 | 0.299–0.307 | 0.391 | 0.296 | 0.260 | 0.402–0.414 | ||||||||||
| Cryptopygus sp. 6, Tasmania | CA7 | 0.002–0.005 (3) | 0.425–0.471 | 0.474–0.479 | 0.403–0.443 | 0.407–0.423 | 0.391–0.399 | 0.385–0.394 | 0.320–0.328 | 0.390–0.411 | 0.259–0.261 | |||||||||
| Cryptopygus antarcticus maximus (Is. Kerguelen) | CAM1 | 0.002–0.012 (4) | 0.140–0.167 | 0.167–0.173 | 0.123–0.160 | 0.322–0.386 | 0.485–0.517 | 0.438–0.476 | 0.362–0.373 | 0.492–0.542 | 0.408–0.430 | 0.401–0.441 | ||||||||
| C. antarcticus maximus (Macquarie I.) | CAM2 | 0.000–0.000 (1) | 0.141–0.165 | 0.174 | 0.134–0.157 | 0.334–0.363 | 0.468 | 0.470 | 0.380 | 0.475–0.491 | 0.406 | 0.413–0.420 | 0.022–0.032 | |||||||
| Cryptopygus antarcticus reagens (I. Crozet) | CAR | 0.002–0.009 (4) | 0.383–0.442 | 0.380–0.402 | 0.393–0.470 | 0.415–0.446 | 0.449–0.479 | 0.425–0.435 | 0.365–0.390 | 0.453–0.499 | 0.316–0.338 | 0.393–0.427 | 0.352–0.405 | 0.378–0.390 | ||||||
| Cryptopygus antarcticus travei (Marion I.) | CAT | 0.000–0.000 (1) | 0.327–0.367 | 0.358 | 0.303–0.351 | 0.315–0.326 | 0.408 | 0.422 | 0.402 | 0.419–0.432 | 0.282 | 0.425–0.429 | 0.389–0.412 | 0.381 | 0.260–0.275 | |||||
| Cryptopygus dubius (Marion I.) | CD | 0.003–0.009 (4) | 0.355–0.403 | 0.378–0.386 | 0.327–0.351 | 0.351–0.379 | 0.395–0.411 | 0.440–0.456 | 0.331–0.339 | 0.406–0.435 | 0.360–0.365 | 0.397–0.383 | 0.406–0.454 | 0.387–0.389 | 0.371–0.415 | 0.324–0.341 | ||||
| Trans-Antarctic Mountains | ||||||||||||||||||||
| Cryptopygus cisantarcticus | CC | 0.009–0.022 (4) | 0.367–0.424 | 0.370–0.391 | 0.357–0.439 | 0.303–0.367 | 0.326–0.369 | 0.289–0.329 | 0.255–0.277 | 0.335–0.391 | 0.279–0.317 | 0.378–0.429 | 0.369–0.419 | 0.355–0.400 | 0.334–0.378 | 0.333–0.374 | 0.333–0.383 | |||
| Gressittacantha terranova | GT | 0.003–0.039 (4) | 0.365–0.392 | 0.403–0.428 | 0.368–0.426 | 0.284–0.301 | 0.398–0.423 | 0.298–0.329 | 0.272–0.288 | 0.397–0.448 | 0.267–0.293 | 0.310–0.345 | 0.393–0.438 | 0.396–0.421 | 0.331–0.404 | 0.372–0.392 | 0.353–0.387 | 0.317–0.360 | ||
| Neocryptopygus nivicolus | NN | 0.002 (2) | 0.357–0.422 | 0.377–0.381 | 0.353–0.370 | 0.376–0.385 | 0.339–0.342 | 0.362–0.366 | 0.318–0.322 | 0.345–0.360 | 0.315–0.319 | 0.336–0.347 | 0.346–0.378 | 0.373–0.377 | 0.409–0.441 | 0.389–0.393 | 0.410–0.418 | 0.333–0.383 | 0.285–0.319 | |
| Antarctophorus subpolaris | AS | 0.003 (2) | 0.395–0.431 | 0.416–0.424 | 0.369–0.400 | 0.372–0.363 | 0.415 | 0.416 | 0.366 | 0.426–0.440 | 0.339–0.349 | 0.367–0.382 | 0.437–0.471 | 0.462 | 0.409–0.444 | 0.314 | 0.347–0.364 | 0.303–0.348 | 0.356–0.390 | 0.309–0.320 |
Rate matrix: A–C = 0.9223, A–G = 5.4335, A–T = 2.8463, C–G = 0.9321, C–T = 8.6194, G–T = 1.0000; I = 0.5069, Γ = 1.1495; base frequencies set to A = 0.2929, C = 0.1899, G = 0.1452, T = 0.3720).
See Supplementary table S1 (Supplementary Material online) for the complete table of sequence divergences (ML and uncorrected) among the ingroup and outgroup taxa).
Number of unique haplotypes (all taxa, all sites) shown in parentheses.
Results
No insertions, deletions, or stop codons were detected in the 586-bp alignment (195 codons), and sequences were most similar to springtail sequences on GenBank. Mean nucleotide composition in this alignment (all taxa, all sites) showed an A–T bias of 61% (A = 26%, T = 35%, C = 21%, G = 18%). Ninety individuals yielded 54 different haplotypes. Among these and the five outgroup taxa there were 317 constant and 269 variable sites. Sequence variability (using a corrected ML model; see Materials and Methods) within each geographic location (<7.0%; table 2) was consistent with conspecific divergences found in other springtails (e.g., Frati, Spinsanti, and Dallai 2001; Stevens and Hogg 2003; Garrick et al. 2004; Hogg and Hebert 2004). There were three cases of low sequence divergence between geographically separated locations (in the subantarctic and maritime regions)—(1) for Cryptopygus antarcticus antarcticus from the three Antarctic Peninsula locations (CAA1, 2, 3) (1.8%–6.3%); (2) for Cryptopygus antarcticus maximus from Îles Kerguelen (CAM1) and Macquarie Island (CAM2) (2.2%–3.2%); and (3) for Cryptopygus sp. 4 from Heard Island (CA5) and Macquarie Island (CA2) (0.7%–1.1%). Sequence divergence was higher (12.3%–47%) between species and subspecies and for the “Cryptopygus antarcticus” group (Cryptopygus sp. 1–6) (table 2).
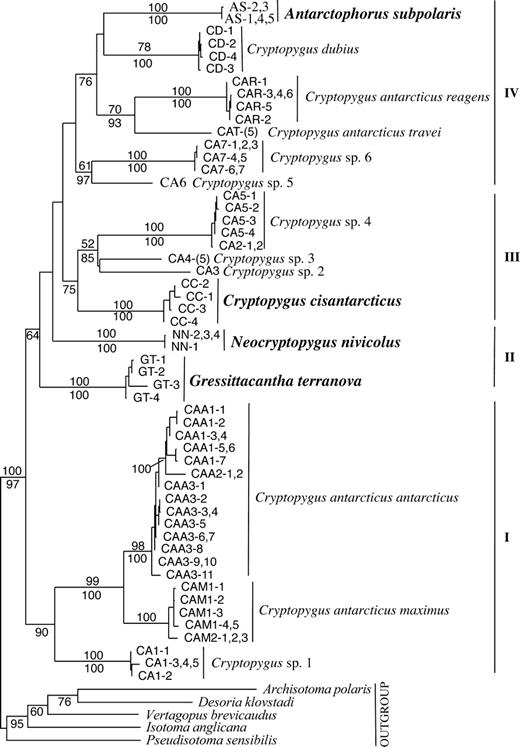
ML analyses were performed on the alignment with the third codon position RY coded. The tree topology was identical to the Bayesian analysis on the original full nucleotide alignment, except that in the latter analysis Gressittacantha terranova (GT) grouped (with only 47% posterior probability) with Neocryptopygus nivicolus (NN). The ML phylogram in figure 2 shows a well-supported (from bootstrap values and posterior probablilities) position of the five outgroup taxa (including the endemic Antarctic Desoria klovstadi). The ingroup comprises four groups. These groups indicate that the four endemic Antarctic species (from the Trans-Antarctic Mountains, fig. 2) do not form a monophyletic group (the unconstrained ML tree [fig. 2] had a lower log-likelihood score compared, using the Shimodaira-Hasegawa test, to a tree with the four species constrained into a monophyletic group), and therefore, are likely to have radiated from more than one evolutionary lineage in Antarctica. Group “I” contains three subgroups (1) C. a. antarcticus (CAA1, 2, 3); (2) C. a. maximus from Îles Kerguelen (CAM1) and Macquarie Island (CAM2); and (3) Cryptopygus sp. 1 from Macquarie Island (CA1) (see fig. 2). Across groups “I” to “IV” several paraphyletic relationships are revealed. Group “II” contains the two Antarctic species G. terranova (GT) and N. nivicolus (NN) as sister taxa in the ML tree but grouped together as a monophyletic sister group to “III” and “IV” in the Bayesian analysis. Within group III the Antarctic Cryptopygus cistantarcticus (CC) is sister to several morphologically recognizable, currently undescribed Cryptopygus species; Cryptopygus sp. 2 from New Zealand (CA3), Cryptopygus sp. 3 from Patagonia (CA4), and Cryptopygus sp. 4 from Heard (CA5) and Macquarie Islands (CA2). Surprisingly, in group IV three Cryptopygus spp.—two from Marion Island (Cryptopygus dubius—CD and Cryptopygus antarcticus travei—CAT) and a single subspecies from the nearby (see fig. 1) Îles Crozet (Cryptopygus antarcticus reagens—CAR)—grouped with the Antarctic species Antarctophorus subpolaris (AS) and were sister to a group containing Cryptopygus sp. 5 from mainland Australia (CA6) and Cryptopygus sp. 6 from Tasmania (CA7) (fig. 2).
ML (GTR + I + Γ) tree for the unique haplotype RY-coded (third codon position only, first and second codon positions were left as nucleotides) alignment of 54 ingroup and 5 outgroup Isotomidae taxa with ML-bootstrap values (500 replicates) indicated above the nodes (for the RY coded) and posterior probabilities below for the Bayesian analysis using a separate model for each codon position on the original full nucleotide alignment (see Materials and Methods). Codes used refer to figure 1 and table 1 (individual codes after location/taxon code; if a taxon is represented by two or more identical haplotypes total number shown in parentheses); bold = the four Trans-Antarctic spp., all other Cryptopygus spp. have a circum-Antarctic distribution, undescribed species (e.g., Cryptopygus sp. 1–6) = “Cryptopygus antarcticus” species group.
Discussion
The high levels of divergence found between Cryptopygus species and related genera was surprising and may indicate divergences for this group in the middle to late Miocene (∼23–5 MYA). However, the range of within-species sequence diversity was remarkable. For example, the paraphyletic lineages and high sequence divergence levels (ML model) greater than 14% between the described and undescribed Cryptopygus species indicate that these lineages have not shared a common evolutionary history or experienced mitochondrial gene flow for ∼23–5.4 Myr. Previous studies have indicated that Cryptopygus is likely to be a complex of undescribed species (Deharveng 1981; Potapov 2001; Rusek 2002; Deharveng, Potapov, and Bedos 2005). Comparisons across groups I–IV between the Cryptopygus spp. and related genera from the Antarctic continent indicate that Cryptopygus is a complex of undescribed species but are also paraphyletic with closer affinities to the Antarctic genera than to C. a. antarcticus (fig. 2). From our results, C. antarcticus sensu stricto (type locality: Gerlache Straits; Antarctic Peninsula) appears restricted to the Antarctic Peninsula and offshore islands (maritime Antarctica) (1.5%–6.3% divergence) (fig. 1 and table 2). The level of sequence divergence (12.3%–17.4%) between populations of C. a. maximus from Îles Kerguelen (CAM1) and Macquarie Island (CAM2) in group I are consistent with levels reported elsewhere for recognized springtail (e.g., >14%; Hogg and Hebert 2004) and other invertebrate species (e.g., Hebert et al. 2003). In fact, the level of sequence divergence found among all the described Cryptopygus subspecies (travei, reagens, and maximus) from C. a. antarcticus indicate that they should be elevated to species status.
In contrast, low levels of sequence divergence were found among C. a. antarcticus individuals from the three Antarctic Peninsula locations (CAA1, 2, 3), most likely the result of isolation during the Pleistocene glacial cycles. Similarly, Macquarie Island is geologically young (above sea level for ∼0.7 to ∼0.5 Myr) and has been colonized recently by at least three independent lineages (1) around 0.55 to 0.3 MYA from Heard Island (Cryptopygus sp. 4); (2) less than 1.6 MYA from Îles Kerguelen (C. a. maximus); and (3) Cryptopygus sp. 1 of unknown origins. In these instances, successful dispersal of springtails is not surprising. For example, Peck (1994) reported transport of many terrestrial arthropods (including springtails) on the sea surface between islands in the Galápagos archipelago, Ecuador. More recently, Moore (2002) reported on several studies that collectively showed springtails can survive both on and in seawater for periods of more than 15 days, and survival could be extended by rafting on/in floating debris. Wind has also been implicated as a long-distance dispersal mechanism for a suite of taxa in the Southern Hemisphere (Greenslade, Farrow, and Smith 1999; Muñoz et al. 2004). Several dispersal pathways/methods are consistent with our molecular data. For example, shallow lineages exist only where suitable ocean currents can act as “dispersal corridors” for springtails, particularly for islands with a relatively recent geological origin (e.g., Macquarie Island 0.7 to 0.5 MYA and Heard Island ∼1 MYA). Marion Island is also young (∼0.3 MYA), but the lineages present suggest a close faunal link (source) with the nearby older Prince Edward Island (18–8 MYA).
Species groups with deeper divergences are likely to have diversified during the middle-late Miocene (23–7 MYA), which agrees with the availability of habitat on Île de la Possession (8.7 MYA), Îles Kerguelen (∼100 MYA), and for the southern temperate and subantarctic groups (Tasmania, mainland Australia, New Zealand, Patagonia, and the Antarctic Peninsula). The deep divergences for the four endemic continental Antarctic species to each other and to other circum-Antarctic lineages also suggest that their origins are likely to be during the Miocene (∼21–11 MYA). We suggest three lines of evidence that indicate the origin and diversification of these Antarctic lineages coincided with the glaciation of the Antarctic continent (23–10 MYA). First, traditional taxonomic descriptions show that the springtail fauna of the Trans-Antarctic Mountains is dominated by 90% endemicity, indicating that they are not recent immigrants (Wise 1967). Second, the Antarctic species are geographically partitioned into four distinct biogeographic regions within the Trans-Antarctic Mountains, suggesting long-term glacial barriers have separated these species assemblages that may have evolved in situ (fig. 1; see also Wise 1967; Stevens and Hogg 2006). This is supported by high levels of sequence divergence (>25%) (table 2) between the Antarctic species and their respective closest phylogenetic neighbor (fig. 2); for example, A. subpolaris (AS) with C. dubius (CD) coincides to isolation around 21–14 MYA (20–15 MYA to each other); the Antarctic G. terranova (GT) to N. nivicolus (NN) around 20–12 MYA; and the Antarctic Cryptopygus cisantarcticus within group III around 18–11 MYA. Successful dispersal may also be restricted for these springtails between local sites in Antarctica (Stevens and Hogg 2002, 2003) and can generally be mapped along meltwater streams and lake margins at microgeographic scales (Nolan et al. 2006), indicating very limited opportunities for dispersal between populations. Finally, molecular evidence for the continental Antarctic species examined here indicate complete isolation from surrounding subantarctic and temperate landmasses once sea/glacial ice was sufficient to restrict successful oceanic dispersal (23–10 MYA). Our molecular dates correlate well with glacio-geological dates of fossils for the isolation and persistence of now-extinct Gondwanan flora and fauna in the Trans-Antarctic Mountains (e.g., Wilson et al. 2002; Ashworth and Kuschel 2003; Ashworth and Preece 2003; Ashworth and Thompson 2003; Lawver and Gahagan 2003; Roberts et al. 2003). Thus, isolation of the Antarctic springtails since the middle-late Miocene (∼21–11 MYA) appears extremely likely.
In summary, the diversification of these springtails is likely to have occurred as a result of the glaciation and isolation of the Antarctic continent completed by ∼10 MYA and not by the sequential break up of Gondwana (completed by ∼32 MYA) or more recently during repeated glacial cycles of the Pleistocene (<2 MYA). Species' distributions and the diversification of these springtails have revealed how global environmental change may have influenced Antarctic biodiversity.
Billie Swalla, Associate Editor
We thank D. Penny, B. Holland, and two anonymous reviewers for helpful comments on the manuscript and W. Allan, B. Holland, and K. Schliep for assistance with analyses, and K. A. J. Wise, P. Convey, and F. Frati for discussions on Antarctic springtails. Many of the collections included in this study were either carried out with the assistance of, or provided by, R. Seppelt, T. G. A. Green, C. Beard, B. J. Sinclair, R. Worland, P. Convey, S. Chown, E. A. Hugo, B. Rocko-Meyer, L. Sancho, K. Green, S. Thiele, D. Bergstrom, K. Kiefer, W. Vincent, and R. Edwards. We are also extremely grateful to D. Penny, B. Gould (University of Waikato Vice-Chancellor's fund), Antarctica New Zealand (K024/28), Australian Antarctic Division (ASAC 2355/2397), National Geographic CRE grant (7790-05), and the NZ Foundation for Research, Science and Technology (MAUX0403) for financial and/or logistic support.
References
Arbogast, B. S., S. V. Edwards, J. Wakeley, P. Beerli, and J. B. Slowinski.
Ashworth, A. C., and G. Kuschel.
Ashworth, A. C., and R. C. Preece.
British Antarctic Survey.
Brower, A. V. Z.
Courtright, E. M., D. H. Wall, R. A. Virginia, L. M. Frisse, J. T. Vida, and W. K. Thomas.
Deharveng, L.
Deharveng, L., M. Potapov, and A. Bedos.
Delsuc, F., M. J. Phillips, and D. Penny.
de Queiroz, A.
Doran, P. T., J. C. Priscu, W. B. Lyons et al. (12 co-authors).
Felsenstein, J.
Folmer, O., M. Black, W. Hoeh, R. Lutz, and R. Vrijenhoek.
Frati, F., G. Spinsanti, and R. Dallai.
Garrick, R. C., C. J. Sands, D. M. Rowell, N. N. Tait, P. Greenslade, and P. Sunnucks.
Gaunt, M. W., and M. A. Miles.
Greenslade, P.
Greenslade, P., R. A. Farrow, and J. M. B. Smith.
Hays, J. D., J. Imbrie, and N. J. Shackleton.
Hebert, P. D. N., S. Ratnasingham, and J. R. de Waard.
Ho, S. Y. W., M. J. Phillips, A. Cooper, and A. J. Drummond.
Hogg, I. D., and P. D. N. Hebert.
Hogg, I. D., and M. I. Stevens.
Lawver, L. A., and L. M. Gahagan.
Lockhart, P. J., M. A. Steel, M. D. Hendy, and D. Penny.
McInnes, S. J., and P. J. A. Pugh.
McLoughlin, S.
Miller, M. F., and M. C. G. Mabin.
Muñoz, J., Á. M. Felicisima, F. Cabezas, A. R. Burgaz, and I. Martinez.
Nolan, L., I. D. Hogg, M. I. Stevens, M. Haase.
Nylander, J. A. A.
Peck, S. B.
Philippe, H., Y. Zhou, H. Brinkmann, N. Rodrigue, and F. Delsuc.
Phillips, M. J., and D. Penny.
Posada, D., and K. A. Crandall.
Potapov, M.
Pugh, P. J. A.
Quek, S.-P., S. J. Davies, T. Itino, and N. E. Pierce.
Rambaut, A., and A. Drummond.
Roberts, A. P., G. S. Wilson, D. M. Harwood, and K. L. Verosub.
Ronquist, F., and J. P. Huelsenbeck.
Rusek, J.
Sanmartin, I., and F. Ronquist.
Stevens, M. I., A. Fjellberg, P. Greenslade, I. D. Hogg, and P. Sunnucks.
Stevens, M. I., and I. D. Hogg.
———.
———.
Sunnucks, P., and D. F. Hales.
Swofford, D. L.
Wharton, D. A.
Willem, V.
Wilson, G. S., J. A. Barron, A. C. Ashworth et al. (22 co-authors).
Author notes
*Allan Wilson Centre for Molecular Ecology and Evolution; Massey University, Palmerston North, New Zealand; ‡Department of Botany and Zoology, Australian National University, Australian Capital Territory, Australia; §Centre for Biodiversity and Ecology Research, University of Waikato, Hamilton, New Zealand; and †School of Biological Sciences and Australian Centre for Biodiversity: Analysis, Policy and Management, Monash University, Clayton, Australia