Abstract
In this work we report a novel scalable strategy to prepare a lithium-air battery electrode from 3D N-doped pierced graphene microparticles (N-PGM) with highly active performance. This approach has combined the merits of spray drying technology and the hard template method. The pierced structured graphene microparticles were characterized physically and electrochemically. An x-ray photoelectron spectrometer and Raman spectra have revealed that the novel structure possesses a higher N-doping level than conventional graphene without the pierced structure. A much higher BET surface area was also achieved for the N-PGM than the conventional N-doped graphene microparticles (N-GM). Cyclic voltammetry indicated that the lithium-air battery with the N-PGM electrode has a better utilization for the graphene mass and a higher void volume for Li2O2 formation than that of the N-GM electrode. N-PGM also exhibits improved decomposition kinetics for Li oxide species yielded in the cathodic reaction. Charge and discharge measurements showed that the N-PGM lithium-air battery achieved an improved specific capacity and an enhanced cycle performance than when an N-GM electrode is used.
Export citation and abstract BibTeX RIS
1. Introduction
Rechargeable lithium-air batteries have received increasing attention as a sustainable energy technology because of their potential to offer three to five times the gravimetric energy density compared with conventional Li-ion batteries [1–3]. However, the real-world commercialization of such devices is still hindered by many issues, from electrochemistry, chemistry, and even to technologies. These issues include the low energy storage efficiency due to high electrochemical polarization corresponding to the charge/discharge voltage distance [4, 5], the poor reversibility, especially at the O2 electrode side [6, 7], the poor stability of electrolytes, which are easily decomposed at both sides [8–10], and the sensitivity to the contamination from the species, such as H2O and CO2, [11–13] etc.
In order to increase the energy storage efficiency and reversibility for the electrochemical reactions within the batteries, much effort has been devoted to exploring highly active electrocatalysts and highly efficient electrodes with ideal structures to meet the requirements for achieving practical applications for Li air batteries. Typically, catalysts are metal oxides [14–19], metal nitrides [20, 21], doped carbon materials [22, 23], and precious metals [24, 25]. These electrocatalysts have all been developed based on the mechanistic hypothesis that they are able to facilitate good reversibility of oxygen reduction reactions (ORR) and oxygen evolution reactions (OER) with an electrochemical polarization that is as low as possible [18]. Among all of the carbon materials, graphene has been attracting particular interest due to its distinctive features, such as high surface area, high electrical conductivity and thermal conductivity, excellent mechanical properties, and flexibility towards functionalization chemistries of functionalities [26–29].
In this paper, we have combined spray drying technology with the hard template method and have successfully prepared pierced textured graphene microparticles with a high surface area. The as-made graphene microparticles have proved to be more highly efficiently doped with N than the conventional porous graphene materials. Furthermore, the obtained N-doped graphene architectures were fully characterized physically and electrochemically, and they showed promising results as potential active electrocatalysts for Li-air batteries.
2. Experimental
2.1. Synthesis of N-PGM
Graphene oxide was synthesized from natural graphite powder (Grade 230, Asbury Carbons) by a modified Hummer method [30]. N-doped pierced graphene microparticles (N-PGM) were obtained according to the following procedure. Briefly, SiO2 solid (particle size: ∼15 nm, Aladdin) and GO suspension (1 mg mL−1) were mixed together in an aqueous suspension with a mass ratio of between SiO2 and GO of 20:80. After being ultrasonically dispersed for 1 h, the aqueous mixture was loaded into a B-290 mini spray drier (Buchi, Swiss) under continuous stirring. The spray-dried product was further heated in a tube furnace under an Ar/NH3 (10%) atmosphere with a heating rate of 10 °C min−1 to 1000 °C for 2 h with a flow rate of 50 ml min−1 to obtain N-doped graphene-encapsulated SiO2 microparticles. The obtained microparticles were then dispersed in diluent HF solution for 1 h to etch out the SiO2. The precipitate was collected by filtering and was then washed with deionized water and ethanol respectively until the filtrate became neutral. The sample was finally dried overnight at 80 °C in a vacuum oven. For comparison, N-doped graphene microparticles (N-GM) without the hard template were also prepared by the spray-drying method and with the same calcination procedure.
2.2. Physical characterizations
The crystalline structure property of N-PGM was examined by x-ray diffraction (XRD) (Rigaku D/MAX-2200/PC). Raman spectra were obtained using a Bruker VERTEX 70. The morphology and microstructure of the spray-dried precursor and N-PGM were investigated using a FEI Nova SEM 230 ultra-high resolution field emission scanning electron microscopy (FE-SEM) (INCA X-Max 80, Oxford Instruments) and transmission election microscopy (TEM) (JEM-2100 F, JEOL Ltd, Japan). Porosity and Brunauer–Emmett–Teller (BET) surface areas of N-PGM were measured using a nitrogen sorption instrument (Micromeritics, ASAP2020). The surface properties of N-PGM were analyzed by x-ray photoelectron spectrometer (XPS) (Kratos Axis Ultra DLD).
2.3. Electrochemical characterizations
The air electrode was prepared using the following procedure. The cathode slurry composed of N-PGM and polytetrafluoroethene (PTFE) in a weight ratio of 90:10 was obtained by uniformly dispersing the materials in ethanol. A lithium metal anode, a glass fiber separator, an as-prepared air electrode, and an electrolyte of 0.1 M LiTFSI (lithium bis-(trifluoromethanesulfonyl)-imide) in TEGDME (tetrathylene glycol dimethyl ether) were employed to assemble the Li-air battery in an argon-filled glove box. For comparison, the Li-air battery with N-GM electrode was also assembled following the same procedure as given above.
The electrochemical performance of the batteries was evaluated in a 1 atm O2 atmosphere using a LAND CT2001A battery testing system at room temperature. Before the galvanostatic discharge/charge measurements, the batteries were placed in flowing pure oxygen for 1 h and then transferred into the oxygen filled glass container for 6 h. The specific capacity and current density were calculated based on the loading amount of graphene material in the electrode.
3. Results and discussion
Spray drying is an ideal approach to produce a dry powder from a liquid solution or suspension which is driven by a hot gas. This method typically leads to homogenous morphology for powder products on a large scale [30]. The preparation of the three-dimensional pierced N-doped graphene has combined the advantages of both spray drying and a hard template, which are that they are general, rapid, low-cost, scalable, and effective. From the SEM and TEM images shown in figures 1(b) and (c), it can be seen that the SiO2 nanoparticles were well mixed with graphene oxide after the spray drying process, which formed well dispersed microparticles. The as-made composites microparticles were calcined under Ar/NH3 (10%) at high temperature to reduce GO into graphene and to introduce N into the graphene structure. The subsequent HF etching removes the SiO2 in the microparticles and results in graphene microparticles that have a three dimensional pierced structure. As shown in figures 1(a) and (d), both the spray-dried precursor and N-PGM product have the precursor and the N-PGM microparticles show irregular but well dispersed morphology, the average particle size is 2 ∼ 3 μm. Figures 1(e) and (f) demonstrate that the nanopores for the N-PGM are quite visible.
Figure 1. SEM (a), (b), (d), (e) and TEM (c), (f) images of spray-dried nano-SiO2/graphene oxide composite before (a), (b), (c) and after the removal of SiO2 hard template (d), (e), (f).
Download figure:
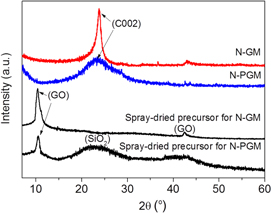
Standard image High-resolution imageIn order to investigate the crystalline properties of the N-PGM and N-GM, and the spray-dried precursors, XRD was performed and the results are shown in figure 2. It is clear that the characteristic peak of GO at about 11° is visible in both the spray-dried precursors of N-PGM and the N-GM. Moreover, the spray-dried precursor of N-PGM shows a broad peak centered at around 2θ = 23°, indicating the low crystallinity of the pore-forming agent of nano-SiO2. For N-PGM and N-GM, the major diffraction peaks match with the characteristic (002) peak of graphite at about 24°, which suggests that GO was efficiently reduced into graphene during the thermal treatment under Ar/NH3 (10%) atmosphere at 1000 °C for 2 h. The characteristic peak of SiO2 is absent in the N-PGM sample, which suggests that most of the nano-SiO2 hard template has been removed. Remarkably, the characteristic (002) peak of N-PGM is much wider than N-GM, which indicates low restacking of graphene sheets in the N-PGM sample. In this way, nano-SiO2 can help the dispersion of graphene sheets during the preparation process of N-PGM.
Figure 2. XRD patterns of spray-dried precursors for both N-PGM and N-GM and the corresponding materials after calcination.
Download figure:
Standard image High-resolution imageThe porosity of the materials was studied by means of the nitrogen sorption technique. The results are shown in figure 3 and the specific values are summarized in table 1. The hysteresis loop at the high (P/P0 = 0.85−1) pressure region in figure 3(a) indicates that large pores from mesopores to macropores are present in the N-PGM sample. While for the N-GM sample (figure 3(b)), the wider hysteresis loop from 0.45 to 1 of relative partial pressure (P/P0) indicates that there is a mesoporous structure in the N-GM sample. Remarkably, the adsorbed quantity of N-PGM is almost an order of magnitude higher than N-GM, which depicts that the N-PGM has a larger surface area and a uniform pore structure. The BET surface areas of N-PGM and N-GM from the N2 absorption measurement are 364.5 and 185.8 m2 g−1, respectively. As shown in the insets in figures 3(a) and (b), N-PGM has an identical pore size distribution with a hierarchically porous structure as its N-GM counterpart, but with a higher average pore diameter and pore volume of ∼21.5 nm and 2.0 cm3 g−1, respectively (versus 5.2 nm and 0.3 cm3 g−1of the N-GM sample, respectively).
Figure 3. Nitrogen adsorption-desorption isotherm and BJH pore size distribution plots (insets) of (a) N-PGM and (b) N-GM.
Download figure:
Standard image High-resolution imageTable 1. BET surface area, pore volume, and average pore width for N-PGM and N-GM, calculated from figure 3.
| Samples | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore width (nm) |
|---|---|---|---|
| N-PGM | 364.5 | 2.0 | 21.5 |
| N-GM | 185.8 | 0.3 | 5.2 |
The XPS spectra in figure 4(a) revealed that the N-doping process occurred for both PGM and GM samples after annealing in an Ar/NH3 (10%) atmosphere. It showed that the N-PGM and N-GM have ∼2.5 at.% and ∼1.9 at.% N, respectively. This information clearly indicates that the pierced structure of graphene microparticles can obtain a higher N-doped level than the conventional structure. As shown in figures 4(b) and 4(c), the N1s peaks in the XPS spectra of N-PGM and N-GM samples were fitted into two peaks, which are pyridinic-N at a lower energy peak of 399.5 eV and quaternary-N at a higher energy peak of 400.6 eV [31]. The ratios of the pyridinic-N/quaternary-N for N-PGM and N-GM samples are calculated to be 1.59 and 2.94, respectively. The smaller ratio of the pyridinic-N/quaternary-N of N-PGM indicates more quaternary N (i.e. N that replaced the carbon atom in the graphene sheets and bonded to three carbon atoms) in the carbon network of N-PGM. It is expected that better catalytic activity may be reflected in the N-PGM samples.
Figure 4. XPS survey spectrum of the N-PGM and N-GM samples (a) and high-resolution XPS spectra of N1s for N-PGM (b) and N-GM samples (c).
Download figure:
Standard image High-resolution imageRaman spectra were recorded to characterize the N-PGM and N-GM, which are shown in figure 5. The peaks of D (∼1355 cm−1), G (∼1600 cm−1), 2D (∼2700 cm−1), and D' (∼2930 cm−1) were observed in the Raman spectra. The intensity ratio of D band and G band (ID/IG) offers useful information to evaluate the degree of crystallinity of graphene. In the present Raman spectra, the ID/IG intensity ratios of N-PGM and N-GM are calculated to be 1.03, and 0.97, respectively, which indicates that the obtained N-PGM and N-GM samples are predominantly hybridized in a sp2 manner, thereby possessing good electronic conductivity. Besides, the value of ID/IG in the N-PGM sample is slightly higher than that of N-GM, indicating that more carbon atoms in the graphene sheets of the pierced structure have been replaced by nitrogen atoms, which is consistent with the results of XPS [32].
Figure 5. Raman spectra of N-PGM and N-GM.
Download figure:
Standard image High-resolution imageThe electrochemical catalytic activity of the electrodes toward ORR and OER has been investigated using cyclic voltammetry (CV) in an oxygen saturated 0.1 M LiTFSI/TEGDME electrolyte. As can be seen from figure 6, both of the electrodes show almost the same onset potential for ORR, which indicates that the pierced textured graphene does not have a different electrochemical kinetic compared with the conventional structure. The ORR for both electrodes peaked at about 2.4 V versus Li+/Li while the N-PGM electrode has a much higher current output than the N-GM electrode. This might be attributed the higher N-doping level and electrochemical active surface area of the pierced structures. Other factors that could contribute are the low re-stacking of graphene sheets, and the higher utilization of available graphene mass and void volume that facilitate the formation of Li2O2 [25]. In the anodic scan, the two peaks at 3.19 V and 3.44 V for the N-GM electrode can be, respectively, assigned to the first two OER pathways according to the elucidated decomposition mechanism that is given below, while the third pathway that occurs at the higher potential region without a clear peak appeared in the CV curves [21]:
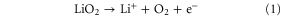
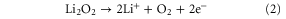
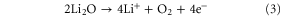
Figure 6. CV curves of N-PGM and N-GM electrodes in the oxygen saturated 0.1 M LiTFSI/TEGDME electrolyte at a scanning rate of 0.5 mV s−1.
Download figure:
Standard image High-resolution imageIn comparison, the two peaks are not distinguishable in the anodic scan for the N-PGM electrode and appear as one peak at 3.20. In addition, the negative shift of the total peaks area related to the OER for the N-PGM electrode compared with that for N-GM electrode obviously depicted the faster reaction kinetics due to the efficient diffusion pathway for O2 product provided by the pierced structure.
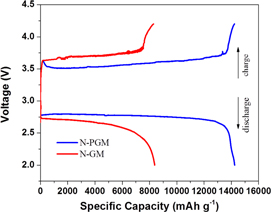
The first discharge/discharge profiles of Li-O2 batteries with two different cathodes at between 2.0 V and 4.25 V are displayed in figure 7. At a current density of 50 mA g−1, the discharge voltage of the N-PGM cell is much higher while the charge voltage is lower than those of the cell with an N-GM electrode. A specific discharge capacity of 14.3 Ah g−1 was obtained when using an N-PGM as a cathode, which is much higher than 8.4 Ah g−1 for the N-GM cell. This performance enhancement reveals that a lower electrochemical polarization and more reaction sites were obtained for the N-doped graphene after pierced texturing.
Figure 7. The first discharge/charge curves of Li-O2 batteries using N-PGM and N-GM as cathodic materials, respectively, at a low current density of 50 mA g−1 within the voltage window between 2.0 and 4.2 V.
Download figure:
Standard image High-resolution imageTo evaluate the cycle performance of the Li-O2 batteries, we carried out cycling tests using a current density of 200 mA g−1 with a fixed capacity of 1000 mAh g−1. From figure 8(a), it can be seen that the distance between the charge and discharge voltage plateaus for the cell with N-PGM electrode showed a slight increase from 1.71 V to 1.84 V after 20 cycles. In comparison, the distance obtained by the cell with N-GM increased from 1.81 V to 2.09 V, which is much higher than that for an N-PGM cell. The results suggest that the cell with an N-PGM electrode has a better cycle performance than when an N-GM electrode is used. In addition, we can also observe at the first charge/discharge cycle that both cells show steady voltage plateaus. However, after five cycles the charge voltage profiles for the N-GM cell kept increasing while the discharge continuously decreased when the cycling approached the fixed capacity of 1000 mAh g−1. This is ascribed to the increase in electrochemical polarization of the N-GM cell. In contrast, the cell with N-PGM maintained steady voltage profiles within the same cycle range.
Figure 8. Voltage profiles of Li-O2 battery using (a) N-PGM and (b) N-GM as cathodic materials at a current density of 200 mA g−1 with a fixed capacity of 1000 mAh g−1 at various cycles.
Download figure:
Standard image High-resolution image4. Conclusions
In conclusion, we have developed an effective protocol to prepare pierced structured graphene using a combination of spray drying technology and the hard template method. The as-prepared graphene based composite was reduced and N-doped in NH3/Ar at high temperature. The pierced structure was confirmed by SEM and TEM. XPS and Raman spectra proved that the pierced structure facilitates the N-doping process to achieve a high N-doping level. CV measurements showed that the N-PGM electrode exhibits similar ORR kinetic to the N-GM electrode with much higher graphene mass utilization, void volume for Li2O2 formation, and faster decomposition kinetic in OER. A specific discharge capacity of 14.3 Ah g−1 was obtained when using N-PGM as a cathode in comparison with 8.4 Ah g−1 for the N-GM lithium-air battery. The battery with an N-PGM electrode has a better cycle performance than that with N-GM electrode, which has been confirmed by the cycling test. This strategy can be used to produce N-PGM on a large scale and could be realized in a continuous process, which suggests a promising potential for the commercial production of electrocatalysts for lithium air batteries.
Acknowledgments
TY and WZ contributed equally to this work. We are grateful for financial support for this work from the National Basic Research Program of China (2014CB239700, 2014CB932303), the Natural Science Foundation of China (21336003, 21403139), China Postdoctoral Science Foundation (2013M541510) and Australian Research Council (DP140100401, FT130100380). This work was also supported by Science and Technology Commission of Shanghai Municipality (14DZ2250800). The authors would also like to thank the Australian National Fabrication Facility (ANFF) for access to equipment.