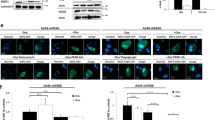
Abstract
Solid tumors meet their demands for nascent blood vessels and increased glycolysis, to combat hypoxia, by activating multiple genes involved in angiogenesis and glucose metabolism. Hypoxia inducible factor-1 (HIF-1) is a constitutively expressed basic helix–loop–helix transcription factor, formed by the assembly of HIF-1α and HIF-1β (Arnt), that is stablized in response to hypoxia, and rapidly degraded under normoxic conditions. It activates the transcription of genes important for maintaining oxygen homeostasis. Here, we demonstrate that engineered down-regulation of HIF-1α by intratumoral gene transfer of an antisense HIF-1α plasmid leads to the down-regulation of VEGF, and decreased tumor microvessel density. Antisense HIF-1α monotherapy resulted in the complete and permanent rejection of small (0.1 cm in diameter) EL-4 tumors, which is unusual for an anti-angiogenic agent where transient suppression of tumor growth is the norm. It induced NK cell-dependent rejection of tumors, but failed to stimulate systemic T cell-mediated anti-tumor immunity, and synergized with B7–1-mediated immunotherapy to cause the NK cell and CD8 T cell-dependent rejection of larger EL-4 tumors (0.4 cm in diameter) that were refractory to monotherapies. Mice cured of their tumors by combination therapy resisted a rechallenge with parental tumor cells, indicating systemic antitumor immunity had been achieved. In summary, whilst intensive investigations are in progress to target the many HIF-1 effectors, the results herein indicate that blocking hypoxia-inducible pathways and enhancing NK-mediated antitumor immunity by targeting HIF-1 itself may be advantageous, especially when combined with cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hockel M et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix Cancer Res 1996 56: 4509–4515
Blancher C, Harris AL . The molecular basis of the hypoxia response pathway: tumor hypoxia as a therapy target Cancer Metast Rev 1998 17: 187–194
Hanahan D, Folkman J . Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis Cell 1996 86: 353–364
Brizel DM et al. Tumor oxygenation predicts the likelihood of distant metastases in human soft tissue sarcoma Cancer Res 1996 56: 941–943
Nordsmark M, Overgaard M, Overgaard J . Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck Radiother Oncol 1996 41: 31–39
Semenza GL, Wang GL . A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation Mol Cell Biol 1992 12: 5447–5454
Maxwell PH, Pugh CW, Ratcliffe PJ . Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism Proc Natl Acad Sci USA 1993 90: 2423–2427
Bunn HF, Poyton RO . Oxygen sensing and molecular adaption to hypoxia Physiol Rev 1996 76: 839–885
Wang GL, Semenza GL . General involvement of hypoxia-inducible factor-1 in transcriptional response to hypoxia Proc Natl Acad Sci USA 1993 90: 4304–4308
Wang GL, Jiang B-H, Rue EA, Semenza GL . Hypoxia-inducible factor 1 is a basic helix–loop–helix PAS heterodimer regulated by cellular O2 tension Proc Natl Acad Sci USA 1995 92: 5510–5514
Jiang BH et al. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1 J Biol Chem 1996 271: 17771–17778
Ratcliffe PJ, O'Rourke JF, Maxwell PH, Pugh CW . Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression J Exp Biol 1998 201: 1153–1162
Semenza GL et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1 J Biol Chem 1996 271: 32529–32537
Dachs GU, Stratford IJ . The molecular response of mammalian cells to hypoxia and the potential for exploitation in cancer therapy Br J Cancer 1996 74: S126–S132
Ryan HE, Lo J, Johnson RS . HIF-1α is required for solid tumor formation and embryonic vascularization EMBO J 1998 17: 3005–3015
Iyer NV et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α Genes Dev 1998 12: 149–162.
Zhong H et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases Cancer Res 1999 59: 5830–5835
Kallio PJ et al. Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin-proteasome pathway J Biol Chem 1999 274: 6519–6525
Huang LE, Gu J, Scha M, Bunn HF . Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway Proc Natl Acad Sci USA 1998 95: 7987–7992
Latif F et al. Identification of the von Hippel-Lindau disease tumor suppressor gene Science 1993 260: 1317–1320
Maxwell PH et al. The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis Nature 1999 399: 271–275
Scheibel T, Buchner, J . The Hsp90 complex-a super-chaperone machine as a novel drug target Biochem Pharmacol 1998 56: 675–682
Minet E et al. Hypoxia-induced activation of HIF-1: role of HIF-1 alpha-Hsp90 interaction FEBS Lett 1999 460: 251–256
Iliopoulos O et al. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein Proc Natl Acad Sci USA 1996 93: 10595–10599
Kaelin WG, Maher ER . The VHL tumor-suppressor gene paradigm Trends Genet 1998 14: 423–426
Kim KJ et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo Nature 1993 362: 841–844
Warren RS et al. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis J Clin Invest 1995 95: 1789–1797
Cheng S-Y et al. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor Proc Natl Acad Sci USA 1996 93: 8502–8507
Kanwar JR et al. Vascular attack by 5,6-dimethylxanthenone-4-acetic acid combined with B7.1-mediated immunotherapy overcomes immune-resistance and leads to the eradication of large tumors Cancer Res (in press)
Kanwar J, Berg R, Lehnert K, Krissansen GW . Taking lessons from dendritic cells: multiple xenogeneic ligands for leukocyte integrins have the potential to stimulate anti-tumor immunity Gene Therapy 1999 6: 1835–1844
Townsend SE, Allison JP . Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells Science 1993 259: 638–370
Chen L et al. Costimulation of anti-tumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4 Cell 1992 71: 1093–1102
Pedley RB et al. Ablation of colorectal xenografts with combined radioimmunotherapy and tumor blood flow-modifying agents Cancer Res 1996 56: 3293–3300
Finlay GJ, Ching LM, Wilson WR, Baguley BC . Resistance of cultured Lewis lung carcinoma cell lines to tiazofurin J Natl Cancer Inst 1987 79: 291–296
Baguley BC, Ching LM . Immunomodulatory actions of xanthenone anticancer agents BioDrugs 1997 8: 119–127
Cao Y et al. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases J Clin Invest 1998 101: 1055–1063
Boehm T, Folkman J, Browder T, O'Reilly MS . Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance Nature 1997 390: 404–407
Melder RJ et al. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium Nat Med 1996 2: 992–997
Ferrara N, Houk L, Jakeman L, Leung DW . Molecular and biological properties of the vascular endothelial cell growth factor family of proteins Endocr Rev 1992 13: 18–32
Borgstrom P, Killan KJ, Sriramarao P, Ferrara N . Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital video microscopy Cancer Res 1996 56: 4032–4039
Saleh M, Stacker SA, Wilks AF . Inhibition of growth of C6 glioma cells in vivo by expression of antisense vascular endothelial growth factor sequence Cancer Res 1996 56: 393–401
Millaruer B et al. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant Nature 1994 367: 576–579
Kayar SR, Archer PG, Lechher AJ, Banchero N . Evaluation of the concentric circles method for estimating capillary-tissue diffusion distances Microvascular Res 1982 24: 342–353
Maxwell PH et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth Proc Natl Acad Sci USA 1997 94: 8104–8109
Acknowledgements
This work was supported in part by grants from the World Health Organization, the Royal Society of New Zealand, the Wellcome Trust (UK), the Cancer Society of New Zealand, the Health Research Council of New Zealand, the Lottery Grants Board of New Zealand, and the Maurice and Phyllis Paykel Trust. We thank Ms Joanna Stewart (Community Health Department, University of Auckland) for help with statistical analyses.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sun, X., Kanwar, J., Leung, E. et al. Gene transfer of antisense hypoxia inducible factor-1 α enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther 8, 638–645 (2001). https://doi.org/10.1038/sj.gt.3301388
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301388
Keywords
This article is cited by
-
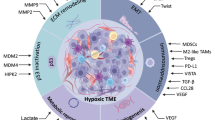
Reprogramming the tumor microenvironment to improve the efficacy of cancer immunotherapies
Medical Oncology (2022)
-
Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy
Journal of Experimental & Clinical Cancer Research (2021)
-
Microvascular bioengineering: a focus on pericytes
Journal of Biological Engineering (2019)
-
HIF-1α-derived cell-penetrating peptides inhibit ERK-dependent activation of HIF-1 and trigger apoptosis of cancer cells under hypoxia
Cellular and Molecular Life Sciences (2019)
-
Cyclin-dependent kinase inhibitor, P276-00, inhibits HIF-1α and induces G2/M arrest under hypoxia in prostate cancer cells
Prostate Cancer and Prostatic Diseases (2012)