Abstract
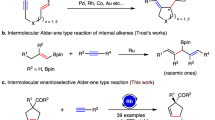
Development of a multicomponent reaction giving a single product is a major goal in synthetic organic chemistry. Catalytic [2+2+2] cyclotrimerization of two distinct alkynes and an alkene with chemo-, regio-, diastereo- and enantiocontrol offers rapid access to highly valued chiral six-membered carbocycles. However, previous [2+2+2]-cyclotrimerization methods have been thwarted by low selectivity and restricted substrate scope. Here we report the rhodium-catalysed [2+2+2] cyclotrimerization of terminal alkynes, alkynoates and cis-enamides to give synthetically valuable chiral cyclohexadienylamines with broad substrate scope and excellent selectivity without the requirement for slow addition or large excesses of reagents. Experimental and theoretical mechanistic studies revealed that the three-component [2+2+2]-cyclotrimerization reaction is highly specific towards use of the cis-enamide.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available in this article and its Supplementary Information (experimental procedures and characterization data). Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under the deposition number CCDC 2068101 ((4R,5S)-(+)-4eaa). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Nunes, P. S. G., Vidal, H. D. A. & Corrêa, A. G. Recent advances in catalytic enantioselective multicomponent reactions. Org. Biomol. Chem. 18, 7751–7773 (2020).
Ahmadi, T., Mohammadi Ziarani, G., Gholamzadeh, P. & Mollabagher, H. Recent advances in asymmetric multicomponent reactions (AMCRs). Tetrahedron Asymmetry 28, 708–724 (2017).
de Graaff, C., Ruijter, E. & Orru, R. V. A. Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev. 41, 3969 (2012).
Shi, S. et al. Three-component radical homo Mannich reaction. Nat. Commun. 12, 1006 (2021).
Boerth, J. A., Maity, S., Williams, S. K., Mercado, B. Q. & Ellman, J. A. Selective and synergistic cobalt(III)-catalysed three-component C–H bond addition to dienes and aldehydes. Nat. Catal. 1, 673–679 (2018).
Ohashi, M., Shirataki, H., Kikushima, K. & Ogoshi, S. Nickel-catalyzed formation of fluorine-containing ketones via the selective cross-trimerization reaction of tetrafluoroethylene, ethylene, and aldehydes. J. Am. Chem. Soc. 137, 6496–6499 (2015).
Zhou, C., Emrich, D. E. & Larock, R. C. An efficient, regio- and stereoselective palladium-catalyzed route to tetrasubstituted olefins. Org. Lett. 5, 1579–1582 (2003).
Suzuki, M., Yanagisawa, A. & Noyori, R. The three-component coupling synthesis of prostaglandins. J. Am. Chem. Soc. 110, 4718–4726 (1988).
Passerini, M. Isonitriles. II. Compounds with aldehydes or with ketones and monobasic organic acids. Gazz. Chim. Ital. 51, 181–189 (1921).
Ugi, I. The α-addition of immonium ions and anions to isonitriles accompanied by secondary reactions. Angew. Chem. Int. Ed. 1, 8–21 (1962).
Tripolitsiotis, N. P., Thomaidi, M. & Neochoritis, C. G. The Ugi three‐component reaction; a valuable tool in modern organic synthesis. Eur. J. Org. Chem. 2020, 6525–6554 (2020).
Wang, S.-X., Wang, M.-X., Wang, D.-X. & Zhu, J. Catalytic enantioselective Passerini three-component reaction. Angew. Chem. Int. Ed. 47, 388–391 (2008).
Zhang, J. et al. Asymmetric phosphoric acid-catalyzed four-component Ugi reaction. Science 361, eaas8707 (2018).
Pla-Quintana, A. & Roglans, A. Chiral induction in [2+2+2] cycloaddition reactions. Asian J. Org. Chem. 7, 1706–1718 (2018).
Domínguez, G. & Pérez-Castells, J. Alkenes in [2+2+2] cycloadditions. Chem. Eur. J. 22, 6720–6739 (2016).
Amatore, M. & Aubert, C. Recent advances in stereoselective [2+2+2] cycloadditions. Eur. J. Org. Chem. 2015, 265–286 (2015).
Galan, B. R. & Rovis, T. Beyond Reppe: building substituted arenes by [2+2+2] cycloadditions of alkynes. Angew. Chem. Int. Ed. 48, 2830–2834 (2009).
Mori, N., Ikeda, S. & Odashima, K. Chemo- and regioselective cyclotrimerization of monoynes catalyzed by a nickel(0) and zinc(II) phenoxide system. Chem. Commun. 181–182 (2001).
Ura, Y. et al. Ruthenium-catalyzed [2+2+2] cycloaddition of three different alkynes. J. Mol. Catal. A 239, 166–171 (2005).
Yamamoto, Y., Ishii, J.-I., Nishiyama, H. & Itoh, K. Ru(II)-catalyzed chemo- and regioselective cyclotrimerization of three unsymmetrical alkynes through boron temporary tether. one-pot four-component coupling via cyclotrimerization/Suzuki–Miyaura coupling. J. Am. Chem. Soc. 126, 3712–3713 (2004).
Heinz, C. & Cramer, N. Synthesis of fijiolide A via an atropselective paracyclophane formation. J. Am. Chem. Soc. 137, 11278–11281 (2015).
Mori, N., Ikeda, S.-I. & Sato, Y. Selective cyclotrimerization of enones and alkynes by a nickel and aluminum catalytic system. J. Am. Chem. Soc. 121, 2722–2727 (1999).
Satoh, Y. & Obora, Y. NbCl3-catalyzed three-component [2+2+2] cycloaddition reaction of terminal alkynes, internal alkynes, and alkenes to 1,3,4,5-tetrasubstituted 1,3-cyclohexadienes. Org. Lett. 13, 2568–2571 (2011).
Komagawa, S. & Saito, S. Nickel-catalyzed three-component [3+2+2] cocyclization of ethyl cyclopropylideneacetate and alkynes—selective synthesis of multisubstituted cycloheptadienes. Angew. Chem. Int. Ed. 45, 2446–2449 (2006).
Hara, H., Hirano, M. & Tanaka, K. Liquid enol ethers and acetates as gaseous alkyne equivalents in Rh-catalyzed chemo- and regioselective formal cross-alkyne cyclotrimerization. Org. Lett. 10, 2537–2540 (2008).
Qiu, Z. & Xie, Z. Nickel-catalyzed three-component [2+2+2] cycloaddition reaction of arynes, alkenes, and alkynes. Angew. Chem. Int. Ed. 48, 5729–5732 (2009).
Kobayashi, M., Suda, T., Noguchi, K. & Tanaka, K. Enantioselective construction of bridged multicyclic skeletons: intermolecular [2+2+2] cycloaddition/intramolecular Diels–Alder reaction cascade. Angew. Chem. Int. Ed. 50, 1664–1667 (2011).
Yoshida, T. et al. Rhodium-catalyzed [3+2+2] and [2+2+2] cycloadditions of two alkynes with cyclopropylideneacetamides. Angew. Chem. Int. Ed. 54, 8241–8244 (2015).
Shibata, Y. & Tanaka, K. Rhodium-catalyzed [2+2+2] cycloaddition of alkynes for the synthesis of substituted benzenes: catalysts, reaction scope, and synthetic applications. Synthesis 44, 323–350 (2012).
Perreault, S. & Rovis, T. Multi-component cycloaddition approaches in the catalytic asymmetric synthesis of alkaloid targets. Chem. Soc. Rev. 38, 3149 (2009).
Hara, J., Ishida, M., Kobayashi, M., Noguchi, K. & Tanaka, K. Highly chemo-, regio-, and enantioselective rhodium-catalyzed cross-cyclotrimerization of two different alkynes with alkenes. Angew. Chem. Int. Ed. 53, 2956–2959 (2014).
Kobayashi, M. & Tanaka, K. Rhodium-catalyzed linear cross-trimerization of two different alkynes with an alkene and two different alkenes with an alkyne. Chem. Eur. J. 18, 9225–9229 (2012).
Shibata, T. et al. Rhodium-catalyzed enantioselective [2+2+2] cycloaddition of diynes with unfunctionalized alkenes. Tetrahedron 63, 12853–12859 (2007).
Aida, Y., Sugiyama, H., Uekusa, H., Shibata, Y. & Tanaka, K. Rhodium-catalyzed asymmetric [2+2+2] cycloaddition of α,ω-diynes with unsymmetrical 1,2-disubstituted alkenes. Org. Lett. 18, 2672–2675 (2016).
Hashimoto, T. et al. The absolute structure of oryzoxymycin. J. Antibiot. 27, 86–87 (1974).
Fukuyama, T. & Kishi, Y. A total synthesis of gliotoxin. J. Am. Chem. Soc. 98, 6723–6724 (1976).
Yeung, H. S. & Corey, E. J. A short enantioselective pathway for the synthesis of the anti-influenza neuramidase inhibitor oseltamivir from 1,3-butadiene and acrylic acid. J. Am. Chem. Soc. 128, 6310–6311 (2006).
Kim, C. U. et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 119, 681–690 (1997).
Lew, W., Williams, M. A., Mendel, D. B., Escarpe, P. A. & Kim, C. U. C3-Thia and C3-carba isosteres of a carbocyclic influenza neuraminidase inhibitor, (3R,4R,5S)-4-acetamido-5-amino-3-propoxyl-1-cyclohexene-1-carboxylic acid. Bioorg. Med. Chem. Lett. 7, 1843–1846 (1997).
Tohmé, R. et al. Direct activation of PP2A for the treatment of tyrosine kinase inhibitor-resistant lung adenocarcinoma. JCI Insight 4, e125693 (2019).
Brazdova, B., Zhang, N., Samoshin, V. V. & Guo, X. Trans-2-aminocyclohexanol as a pH-sensitive conformational switch in lipid amphiphiles. Chem. Commun. 4774 (2008).
Hernandez, L. W., Klöckner, U., Pospech, J., Hauss, L. & Sarlah, D. Nickel-catalyzed dearomative trans-1,2-carboamination. J. Am. Chem. Soc. 140, 4503–4507 (2018).
Masutomi, K., Sugiyama, H., Uekusa, H., Shibata, Y. & Tanaka, K. Asymmetric synthesis of protected cyclohexenylamines and cyclohexenols by rhodium-catalyzed [2+2+2] cycloaddition. Angew. Chem. Int. Ed. 55, 15373–15376 (2016).
Shimizu, H., Nagasaki, I. & Saito, T. Recent advances in biaryl-type bisphosphine ligands. Tetrahedron 61, 5405–5432 (2005).
Marcone, J. E. & Moloy, K. G. Kinetic study of reductive elimination from the complexes (diphosphine)Pd(R)(CN). J. Am. Chem. Soc. 120, 8527–8528 (1998).
Tanaka, K., Toyoda, K., Wada, A., Shirasaka, K. & Hirano, M. Chemo- and regioselective intermolecular cyclotrimerization of terminal alkynes catalyzed by cationic rhodium(I)/modified BINAP complexes: application to one-step synthesis of paracyclophanes. Chem. Eur. J. 11, 1145–1156 (2005).
Dyker, G. Palladium-catalyzed C-H activation of tert-butyl groups: a simple synthesis of 1,2-dihydrocyclobutabenzene derivatives. Angew. Chem. Int. Ed. 33, 103–105 (1994).
Dyker, G. Palladacycles as reactive intermediates. Chem. Ber. 130, 1567–1578 (1997).
Gimalova, F. A. et al. Effect of the β-substituent with respect to the azido group on the reactivity of methyl (2E)-3-[5-(azidomethyl)-2,2-diethyl-1,3-dioxolan-4-yl]-2-methylprop-2-enoate. Russ. J. Org. Chem. 49, 1047–1054 (2013).
Maeda, S., Ohno, K. & Morokuma, K. Systematic exploration of the mechanism of chemical reactions: the global reaction route mapping (GRRM) strategy using the ADDF and AFIR methods. Phys. Chem. Chem. Phys. 15, 3683–3701 (2013).
Roglans, A., Pla-Quintana, A. & Solà, M. Mechanistic studies of transition-metal-catalyzed [2+2+2] cycloaddition reactions. Chem. Rev. 121, 1894–1979 (2021).
Sakaki, S., Kitaura, K. & Morokuma, K. Structure and coordinate bonding nature of nickel(0) and copper(I) carbon dioxide complexes. An ab initio molecular orbital study. Inorg. Chem. 21, 760–765 (1982).
Komagawa, S., Wang, C., Morokuma, K., Saito, S. & Uchiyama, M. Mechanistic origin of chemo- and regioselectivity of nickel-catalyzed [3+2+2] cyclization reaction. J. Am. Chem. Soc. 135, 14508–14511 (2013).
Evans, M. G. & Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 31, 875–894 (1935).
Acknowledgements
This research was supported partly by Grants-in-Aid for Scientific Research (number JP19H00893 to K.T., number 17H06173 to M.U. and numbers JP20H04661 and JP18H04504 to H.U.) from JSPS (Japan) and CREST (number JPMJCR19R2 to M.U.) from JST (Japan). We thank Takasago International Corporation for the gift of Segphos and H8-BINAP, and Umicore for the support in supplying the rhodium complexes. Allotment of computational resources from and HOKUSAI BigWaterfall (Project G19012, RIKEN) and TSUBAME (Tokyo Institute of Technology) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
K.F. designed the project, conducted experimental studies and wrote a draft of the experimental studies. K.M. conducted preliminary experiments. Y.N., T.S., J.K. and M.U. conducted computational studies and wrote a draft of the computational studies. H.S. and H.U. performed the X-ray crystal structure analysis. K.T. designed, advised and directed the project, and wrote the manuscript. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Martin Kotora and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Thomas West was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Materials and methods, synthetic experiments, single-crystal X-ray diffraction analysis of (+)-4eaa, computational studies, NMR spectra, HPLC data, references, Figs 1–7 and Tables 1–8.
Supplementary Data 1
Crystal structure of +4eaa; CCDC 2068101.
Rights and permissions
About this article
Cite this article
Fujii, K., Nagashima, Y., Shimokawa, T. et al. Stereoselective cyclohexadienylamine synthesis through rhodium-catalysed [2+2+2] cyclotrimerization. Nat. Synth 1, 365–375 (2022). https://doi.org/10.1038/s44160-022-00043-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00043-2
This article is cited by
-
Cyclotrimerization takes orders from rhodium
Nature Synthesis (2022)