Abstract
Isoprene, C5H8, inserts about half of the non-methane carbon flux of biogenic origin into the atmosphere. Its degradation is primarily initiated by the reaction with hydroxyl radicals. Here we show experimentally the formation of reactive intermediates and corresponding closed-shell products from the reaction of hydroxyl radicals with isoprene for low nitric oxide and low hydroperoxy radical conditions. Detailed product analysis is achieved by mass spectrometric techniques. Quantum chemical calculations support the usefulness of applied ionization schemes. Observed peroxy radicals are the isomeric HO-C5H8O2 radicals and their isomerization products HO-C5H8(O2)O2, bearing most likely an additional hydroperoxy group, and in traces HO-C5H8(O2)2O2 with two hydroperoxy groups. Main closed-shell products from unimolecular peroxy radical reactions are hydroperoxy aldehydes, C5H8O3, and smaller yield products with the composition C5H8O4 and C4H8O5. Detected signals of C10H18O4, C10H18O6, and C5H10O2 stand for products arising from peroxy radical self- and cross-reactions.
Similar content being viewed by others
Introduction
Hydrocarbons with biogenic origin account for about 90% of the total volatile organic compounds emitted into Earth’s atmosphere1. Isoprene represents the most abundant non-methane hydrocarbon in this process with an estimated emission rate of about 600 × 106 metric tons of carbon per year2. The emission rate may be altered in the future due to changes of environmental conditions3.
Isoprene’s degradation process is initiated by the reaction with atmospheric oxidants where the reaction with OH radicals is distinctly dominant4. The subsequent oxidation pathways and formed products influence the tropospheric chemistry, especially in isoprene-dominated areas such as in the tropics or other large forestlands5,6,7. A mechanistic understanding of the OH + isoprene reaction in the atmosphere has already been the subject of a large number of investigations up to now and the progress is tightly connected to the development of analytical techniques and the improvement of theoretical calculations8.
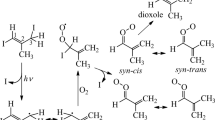
OH attack of the conjugated diene system may occur at the four different diene carbon atoms with a preference of terminal OH addition forming allyl radicals9. Subsequently, reversible O2 addition to the allyl system generates β- and δ-HO-C5H8O2 radicals10,11. The reversibility of the O2 addition makes an interconversion of the different RO2 radical isomers possible. Available room-temperature rate coefficients of HO-C5H8O2 radical decomposition to the corresponding allyl radicals and O2 are in the range of 0.018–4 s−112 or 0.14–16 s−113, indicating that about 1 min12 or 10 s13 are needed to equilibrate the HO-C5H8O2 radical distribution. Observations of HO-C5H8O2 radicals have been previously reported using either UV absorption measurements for elevated RO2 radical concentrations14 or detection by mass spectrometry in a low-pressure experiment at 1–2 Torr He15. Figure 1 shows a scheme of the first reaction steps from OH attack at the 1-position, which is based on the current knowledge in the literature11,12,13. The products from this reaction are marked with “I”. The formation of the analogous products starting from OH attack at the 4-position, marked with “II”, is depicted in Supplementary Fig. 1. OH attack in 2- and 3-position accounts for ≤ 5% of the initial OH adduct distribution each and will not be further considered here8,9,11,16. Possible bimolecular RO2 radical reactions with NO, HO2, or other RO2 radicals have been omitted to keep the schemes as lucid as possible, drawing the attention to the unimolecular RO2 reactions. As a result of theoretical calculations, Peeters et al.11 first suggested RO2 isomerization steps that are competing with the well-established bimolecular RO2 radical reactions in the atmosphere. According to that work, fast 1,6 H-shift of the Z-δ-HO-C5H8O2 radicals forming hydroxy–hydroperoxy allyl radicals was predicted with rate coefficients higher than 1 s−111, which were later revised to somewhat lower values12. Continuous reproduction of the Z-δ-HO-C5H8O2 radicals from the whole RO2 radical reservoir via RO2 interconversion enables the 1,6 H-shift step to become an important exit channel in the case of less efficient bimolecular RO2 reactions. The RO2 radical resulting from O2 addition at the α-position to the OH group of the hydroxy–hydroperoxy allyl radical, HO-C5H8(O2)O2_1 I in Fig. 1, rapidly decomposes forming an unsaturated hydroperoxy aldehyde, HPALD I, along with HO211,17. Experimental support for HPALD formation came from chamber experiments13,18 and from a flow tube study19.
First reaction steps starting from the OH attack at the 1-position. The given scheme is in line with the current knowledge given in the literature11,12,13. Boxes indicate the products whose corresponding signals have been observed by mass spectrometry. Yellow: RO2 radicals; blue: closed-shell products. Possible bimolecular RO2 radical reactions are not shown. Corresponding reaction scheme for the OH attack at the 4-position is given in Supplementary Fig. 1
O2 addition at the γ-position relative to the OH group forms an RO2 radical, HO-C5H8(O2)O2_2 I, which very rapidly undergoes an enolic 1,6 H-shift20 generating an alkyl radical. The alkyl radical is believed to either add O2 resulting in the next RO2 radical, HO-C5H8(O2)2O2 I, or to decompose forming an epoxy hydroperoxy carbonyl, C5H8O4 I, along with an OH radical12,13. It should be noted that there is no proof for the epoxide structure of C5H8O4 I proposed by Teng et al.13. Decomposition of the RO2 radical, HO-C5H8(O2)2O2 I, is expected to lead to a dihydroperoxy carbonyl, C4H8O5 I, after H-shift reactions and subsequent elimination of CO and an OH radical12. Experimental evidence for the formation of a C4H8O5 product is missing up to now. On the other hand, Teng et al.13 observed a product signal by means of mass spectrometry being in line with the composition of C5H8O4. It should be noted that 1,5 H-shift isomerization of the β-OH-C5H8O2 radicals has been also discussed as a result of theoretical calculations11,12,21. The predicted rate, however, is relatively small, making 1,5 H-shift steps less important for the product formation of OH + isoprene.
Here we report on the direct probing of the initially formed RO2 radicals and closed-shell products from the atmospheric reaction of OH radicals with isoprene conducted in a flow system22,23. With the exception of runs in the presence of NO, RO2 radical consumption by bimolecular reactions were less important due to the kinetic limitation for a reaction time of 7.9 s or less and the low concentrations of RO2 radicals and possible co-reactants. Hence, RO2 isomerization steps and the resulting product formation were observable for conditions of less important bimolecular RO2 radical reactions. The results provide experimental-based insight into the first reaction steps of the OH + isoprene reaction with special attention to RO2 isomerization. This, together with other recent developments12,13, allows a more exact description of isoprene’s oxidation pathways in atmospheric modeling.
Results
Detected products from OH + isoprene
Efficient mass spectrometric detection of RO2 radicals and closed-shell products is achieved by atmospheric pressure ionization using aminium, i.e., protonated n-propyl-, ethyl- or methylamine, or hydrazinium, i.e., protonated hydrazine, as the reagent ions. n-Propyl-aminium was already applied in a former study for the detection of RO2 radicals and other oxidized products23; the others are used here for the first time. For comparison, analysis was carried out with acetate22,24,25,26 and iodide27,28 as well. The reaction products were observed as clusters with the respective reagent ion as well as deprotonation products in the case of acetate. Figure 2 shows cluster ion traces from a typical experiment of the OH + isoprene reaction using OH radical production via ozonolysis of tetramethylethylene (TME). It should be noted that OH radical formation via O3 + TME is directly associated with the formation of acetonyl peroxy radicals, CH3C(O)CH2O2, representing additional RO2 radicals in the reaction system23,29.
Signals of selected cluster ion traces from the OH + isoprene reaction. Cluster ion traces attributed to C5H8O3, C5H8O4, C4H8O5, and HO-C5H8(O2)αO2, α = 0, 1, and 2 are shown depending on the reaction conditions. OH radicals were mainly generated via tetramethylethylene (TME) ozonolysis. Product ionization was carried out by means of hydrazinium, H2NNH3+. The background measurement has been done in the presence of isoprene. Ozone was switched on at measurement cycle 13 and TME at measurement cycle 46 starting the main OH generation. It is noteworthy that the (C4H8O5)H2NNH3+ signal was influenced by other ions, which probably arise from OH reactions with background impurities, being not corrected here. Reactant concentrations are [O3] = 9.4 × 1011, [TME] = 2.0 × 1011, and [isoprene] = 2.5 × 1012 molecules cm−3 and the reaction time 7.9 s. Stated concentrations represent lower limits; a measurement cycle comprises 60 s data accumulation
Signals of the corresponding masses for the closed-shell products C5H8O3 (HPALDs), C5H8O4, and C4H8O5, as well as for the RO2 radicals HO-C5H8(O2)αO2, α = 0, 1, and 2, are identified qualitatively in line with the expected product distribution based on recent theoretical and experimental work12,13, see Fig. 1. Signals of the most abundant products HO-C5H8O2, C5H8O3, and C5H8O4 are already visible from the pure O3 + isoprene reaction accounting for 5–6% of the maximum signal obtained under conditions of the main OH generation via O3 + TME. This behavior is in line with the results from modeling, indicating a 5% fraction of OH radicals produced via O3 + isoprene in the O3/TME/isoprene system (see the reaction scheme in Supplementary Note 1). There are no indications that signals from pure isoprene ozonolysis influence the product signals attributed to the OH + isoprene reaction. Results from experiments with labeled isoprene, isoprene-1-13C, also confirm the signal assignment based on the signal shift by 1.003 Th.
Other product signals attributed to OH + isoprene products, not given in Fig. 2, indicate the formation of the isomeric hydroxy hydroperoxides HO-C5H8OOH and probably other C5H10O3 products, and the accretion products C10H18O4 and C10H18O6. The product with the composition C5H10O2 is ascribed to HO-C5H8OH formed from HO-C5H8O2 radical dismutation reactions.
Further support especially for the ascertained RO2 radicals comes from runs applying photolysis of isopropyl nitrite as a second OH radical source, i.e., with OH radical production via NO + HO230. Under these conditions, the RO2 radicals are reacting with NO forming organic nitrates RONO2 with a yield of up to 0.331. The expected RONO2 signals of the three different HO-C5H8(O2)αO2 radicals, α = 0, 1, and 2, are visible but with a very weak signal for the highest oxidized RONO2 arising from the RO2 radical with α = 2 (Supplementary Fig. 2). Signals of the three RO2 radicals and for the closed-shell products featured roughly the same relative abundance among each other as observed in the runs with TME ozonolysis for OH radical production.
Efficiency of product detection and bimolecular pathways
The lack of needed reference substances or of an independent way of defined in-situ product formation makes signal calibration very challenging, especially for RO2 radicals32. Thus, calibration factors are calculated taking into account the following: (i) collision limit of the cluster formation rate from the ion-molecule reaction22,23,24,33,34,35,36, i.e., reagent ion + product → (product)reagent-ion cluster or via the ligand switch reaction (X)reagent ion + product → (product)reagent-ion cluster + X (X stands for a ligand) and (ii) negligible cluster losses inside the instrument. Concentrations calculated in this way represent lower limits. It is impossible to check the validity of condition (i). However, quantum chemical calculations on the cluster stability were performed to get a measure for the probability that a formed cluster survives the different stages in the mass spectrometer without decomposition as requested in condition (ii).
It is discovered that the different aminium reagent ions and hydrazinium are able to form two hydrogen bonds to all of the products investigated here (see examples in Fig. 3). It is worth noting that all products are bearing at least two oxygen-containing moieties (see Fig. 1). For a selected oxidation product, binding with the reagent ion becomes stronger in the following order: n-propyl-aminium < methyl-aminium < hydrazinium. The corresponding clusters formed by iodide as the reagent ion show a distinctly lower stability being qualitatively in line with the experimentally observed lower detection sensitivities in the case of iodide. On the other hand, for acetate the calculations predict strongly bound (product)acetate clusters. The measurements, however, reveal that acetate is a less efficient reagent ion for product detection in this reaction system probably due to a cluster formation rate that is distinctly lower than collision limit, i.e., condition (i) is probably not fulfilled. Calculated formation enthalpies and free energies of reagent-ion clusters with Z-δ- and β-OH-C5H8O2 radicals, C5H8O3 (HPALDs), and C4H8O5 are given in Supplementary Tables 1 and 2.
Product measurements with the six reagent ions (n-propyl-, ethyl- or methyl-aminium, hydrazinium, acetate, and iodide) were conducted using a measurement series with TME ozonolysis for OH radical formation varying the ozone concentration in the range (1.2–95) × 1010 molecules cm−3 for constant TME and isoprene concentrations of 2.0 × 1011 and 2.5 × 1012 molecules cm−3, respectively. The amount of reacted isoprene increased linearly with rising ozone and subsequently rising OH radical concentrations, reacted isoprene = (1.7–132) × 107 and steady-state OH concentration = (8.5–670) × 103 molecules cm−3 as calculated from a simple reaction scheme. Consecutive OH radical reactions of the first-generation products, which consumed < 0.03% of formed products, can be neglected (see Supplementary Note 1).
HO-C5H8(O2)αO2 with α = 0–2 and C5H8O3 (HPALDs)
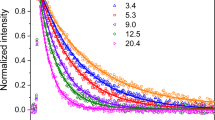
Figure 4 shows the obtained lower limit concentrations of the isomeric RO2 radicals HO-C5H8(O2)αO2, α = 0, 1, and the HPALDs C5H8O3. Considering the three aminium reagent ions and hydrazinium, the detection sensitivity behaves in the following order: n-propyl-aminium < ethyl-aminium ≤ methyl-aminium ≤ hydrazinium in line with the trend of the cluster stability from quantum chemical calculations (Supplementary Tables 1 and 2).
Lower-limit product concentrations obtained by different ionization schemes. Section a shows the results of the HO-C5H8O2 radicals, section b the HO-C5H8(O2)O2 radicals, and section c the data for C5H8O3 (HPALDs). In section c, the blue dashed-dotted line represents the calculated HPALD data and the blue dotted line the total amount of HPALD and “MW 116,” based on Teng et al.13 using their reaction mechanism and the stated 25% HPALD fraction of the total 1,6 H-shift products13 (see Supplementary Note 2). Reactant concentrations are [O3] = (1.2–95) × 1010, [TME] = 2.0 × 1011 and [isoprene] = 2.5 × 1012 molecules cm−3 and the reaction time 7.9 s. Calculated steady-state OH concentrations ranged from 8.5 × 103 to 6.7 × 105 molecules cm−3
In the case of the isomeric HO-C5H8O2 radicals (Fig. 4a), the sensitivity differences are distinctly marked in the expected order. Even by applying hydrazinium as the reagent ion, obtained HO-C5H8O2 radical concentrations are by a factor of about 15 smaller than the calculated HO-C5H8O2 radical concentration from a detailed reaction mechanism. The calculations consider 1,6 H-shift product formation based on the RO2 radical dynamics given by Teng et al.13 and the HO-C5H8O2 + HO2 reaction (see Supplementary Note 2). The HO-C5H8O2 radicals are bearing only an OH group besides the peroxy moiety and the cluster formation enthalpy of β-HO-C5H8O2 radicals, representing the main HO-C5H8O2 fraction12,13, is relatively small compared with the other products (Supplementary Tables 1 and 2). Insufficient cluster stability is most likely the reason for the relatively low detection sensitivity. In this context, NH4+ ionization appears to be a more efficient way of HO-C5H8O2 radical detection caused by the expected higher (HO-C5H8O2)NH4+ cluster stability23,36. The measurements using iodide and acetate for ionization yield lower values, especially in the latter case with signal intensities close to the detection limit.
The HO-C5H8(O2)O2 lower limit concentrations (Fig. 4b) are within a factor of about two using either hydrazinium, methyl- or ethyl-aminium, or iodide in the ionization process. The good agreement of the results allows the conclusion that HO-C5H8(O2)O2 radicals are measured with close to maximum sensitivity applying these four reagent ions, i.e., the given lower limit concentrations are approaching the “real” concentrations. The additional functional group, most likely an OOH group, causes enhanced detectability of HO-C5H8(O2)O2 radicals due to stronger binding to the reagent ions.
Only very weak signals attributed to HO-C5H8(O2)2O2 radicals are visible being close to the background level, especially in the case of ionization by the aminium ions and hydrazinium. The resulting lower limit concentrations do not exceed concentrations of 2 × 105 molecules cm−3 (Supplementary Fig. 3). It is assumed that further RO2 radical functionalization gives rise to the formation of a second OOH group in HO-C5H8(O2)2O2 (Fig. 1 and Supplementary Fig. 1), which causes good detectability also by means of acetate and iodide with close to maximum sensitivity.
The lower limit concentrations obtained for C5H8O3, HPALDs, agree well within a factor of 2–3 using hydrazinium or the three aminium ions for ionization (see Fig. 4c). Our signal measured at the exact mass of the (C5H8O3)reagent-ion cluster is solely attributed to the HPALDs. Also here, close to maximum sensitivity can be expected especially in the case of hydrazinium and methyl-aminium used as the reagent ions. Further support for efficient HPALD detection comes from the comparison with the data by Teng et al.13, which account for about half of our maximum HPALD concentrations (see the blue dashed-dotted line in Fig. 4c). Based on end-product analysis in combination with theoretical results, the work by Teng et al.13 provides comprehensive information on the RO2 radical dynamics and the HO-C5H8O2 isomer-specific product formation needed for reasoned modeling of the reaction system. These authors also reported other, nonspecified “MW 116” products with a 0.61 yield relative to the HPALDs13, which could have an impact on our HPALD analysis if formed under our conditions. Unfortunately, there is no information on the chemical structure of these “MW 116” products and possible pathways leading to these compounds13. Assuming that the exact mass of “MW 116” is consistent with the chemical composition C5H8O3, the formation of the first-generation products other than unsaturated C5 hydroperoxy carbonyls (HPALDs) is mechanistically hard to explain. The total amount of HPALD and “MW 116” by Teng et al.13 accounts for up to 80% of our HPALD concentrations measured with hydrazinium ionization (see the blue dotted line in Fig. 4c). Other experimental data on non-isomer-specific HPALD formation18,19 are not directly comparable because of the different bimolecular HO-C5H8O2 reactivity and HO-C5H8O2 isomer distribution under the different reaction conditions.
C5H8O4 and C4H8O5
Measurement data for the closed-shell products C5H8O4 and C4H8O5 from 1,6 H-shift reactions of the Z-δ-HO-C5H8O2 radicals are given in the Supplementary Figs. 4 and 5, respectively. Based on the current understanding of the reaction pathways and the expected product structures, C5H8O4 and C4H8O5 are bearing at least one OOH group and a carbonyl moiety, and possess a similar molecular structure to the HPALDs. The assumed detectability of C5H8O4 and C4H8O5 with close to maximum sensitivity, such as for the HPALDs, is supported by the strong binding with the reagent ions, as calculated in the case of C4H8O5 I (Supplementary Tables 1 and 2).
C5H10O3
Product formation in the reaction system is inevitably connected with HO2 radical generation, especially from HPALD production. A further HO2 radical source is the O3 + isoprene reaction37 and additionally we assume an 8% HO2 yield from O3 + TME accounting for the pathways not leading to OH radical production (OH yield: 92%38). The expected signal of C5H10O3, attributed to hydroxy hydroperoxides from the HO-C5H8O2 + HO2 reaction via pathway (1), was observed (see Supplementary Fig. 6).
Measurements with hydrazinium and methyl-aminium yielded again almost identical results. The comparison with calculated HO-C5H8OOH concentrations agreed reasonably with the measurement only for the highest isoprene conversion with [O3] = 9.5 × 1011 molecules cm−3, calculated C5H10O3 concentration: 1.6 × 107 and maximum C5H10O3 measurement: 3.9 × 107 molecules cm−3. Apart from that, the calculations clearly underpredict the measurements. This behavior points to other HO2 radical sources, not considered yet, or probably to the formation of C5H10O3 substances other than the hydroxy hydroperoxides. Formation of the second-generation products, such as the dihydroxy epoxides39, can be neglected due to the small isoprene conversion.
C5H10O2, C10H18O4, C10H18O6 and other accretion products
Although the reaction conditions were chosen in such a way that the RO2 radical consumption by bimolecular steps is less important for the RO2 balance, reaction products from RO2 self- and cross-reaction become visible, especially for conditions of relatively high isoprene conversion. Figure 5 shows the lower limit concentrations of C5H10O2 and C10H18O4 along with the HO-C5H8O2 radicals measured by hydrazinium ionization and Supplementary Fig. 7 shows the C5H10O2 measurement data obtained from the different reagent ions. The concentrations of C5H10O2 and C10H18O4 show a parallel behavior and their slope with rising ozone, and subsequently rising isoprene conversion, is almost twice the slope of the HO-C5H8O2 radical concentration (Fig. 5). This behavior is consistent with a bimolecular RO2 radical reaction leading to C5H10O2 and C10H18O4. It can be assumed that C5H10O2 mainly stands for the diol HO-C5H8OH formed via RO2 radical dismutation reactions31,
where R′O2 represents either an HO-C5H8O2 radical or an acetonyl peroxy radical CH3C(O)CH2O2. Acetonyl peroxy radicals are formed in the O3 + TME reaction together with the desired OH radical generation23,29. Ruppert and Becker40 reported the formation of two unsaturated C5 diols, 2-methyl-3-butene-1,2-diol and 3-methyl-3-butene-1,2-diol, with a total molar yield of 7.1 ± 2.3% from the OH + isoprene reaction conducted in a chamber for low NOx conditions. The distinctly lower diol yield of < 0.5% from the present study is due to the less efficient bimolecular RO2 radical reactions under our experimental conditions. Unambiguous identification of the corresponding carbonyl products R′(–H, = O) fails because of the large background signals in the respective range of the mass spectrum. The HO-C5H8O2 radical self-reaction forms the accretion product C10H18O4 according to the general accretion pathway RO2 + R′O2 → ROOR′ + O223.
Lower limit C5H10O2 and C10H18O4 concentrations compared with the precursor RO2 radicals. Reactant concentrations are [O3] = (1.2–95) × 1010, [TME] = 2.0 × 1011 and [isoprene] = 2.5 × 1012 molecules cm−3 and the reaction time 7.9 s. Analysis has been carried out by hydrazinium ionization. HO-C5H8O2 radical concentrations are underestimated by a factor of about 15
The rate coefficient k3 = 6 × 10−13 cm3 molecule−1 s−1, T = (297 ± 1) K, has been estimated in a previous study assuming an uncertainty to be not higher than a factor of 2–323. The measured C10H18O4 concentrations at the residence time t are used to count back the HO-C5H8O2 radical concentrations according to [HO-C5H8O2]t = (3 [C10H18O4]t/t/k3)0.523, which are found in good agreement with the calculated HO-C5H8O2 data from a detailed reaction mechanism based on the RO2 radical dynamics given by Teng et al.13, see Supplementary Note 2 and Supplementary Fig. 8.
For elevated isoprene conversion with [O3] > 1012 molecules cm−3 also the formation of C10H18O6 from the RO2 cross-reaction via pathway (4) becomes detectable (see Fig. 6).
Accretion product formation from the extended range of isoprene conversion. Reactant concentrations are [O3] = (1.2–22) × 1011, [TME] = 2.0 × 1011, and [isoprene] = 2.5 × 1012 molecules cm−3 and the reaction time 7.9 s. Analysis has been carried out by hydrazinium and ethyl-aminium ionization. C10H18O4 is formed from the self-reaction of HO-C5H8O2 and C10H18O6 from the cross-reaction with HO-C5H8(O2)O2, C8H14O4,6 from the cross-reactions of CH3C(O)CH2O2 with HO-C5H8(O2)αO2, α = 0, 1, and C6H10O4 from the CH3C(O)CH2O2 self-reaction. CH3C(O)CH2O2 radicals are formed from TME ozonolysis
Further observed accretion products are C8H14O4 and C8H14O6 from the cross-reaction of acetonyl peroxy radicals, CH3C(O)CH2O2, with HO-C5H8(O2)αO2, α = 0, 1, as well as C6H10O4 from the CH3C(O)CH2O2 self-reaction (Fig. 6). The very good agreement between the results using either hydrazinium or ethyl-aminium ionization suggests that also the accretion products are detected with close to maximum sensitivity.
HO-C5H8O2 radical balance. Modeling of the reaction system for [O3] = 9.5 × 1011, [TME] = 2.0 × 1011 and [isoprene] = 2.5 × 1012 molecules cm−3, and a reaction time 7.9 s reveals an isoprene conversion of 1.322 × 109 and the concentration of the isomeric HO-C5H8O2 radicals of 1.265 × 109 molecules cm−3. Calculations were performed based on the data by Teng et al.13 for the HO-C5H8O2 radical dynamics and the 1,6 H-shift reactions including HO-C5H8OOH formation via pathway (1) (see the reaction mechanism in Supplementary Note 2). Using the results with hydrazinium ionization, total concentrations of 1,6 H-shift products and HO-C5H8OOH, assuming that C5H10O3 solely stands for the hydroxy hydroperoxides, account for 6.8 × 107 molecules cm−3 in reasonable agreement with the modeling results. Additional HO-C5H8O2 consuming steps are the accretion product formations with 3.5 × 107 molecules cm−3 in total (mainly C8H14O4 formation from the reaction with CH3C(O)CH2O2 radicals) as well as the dismutation reaction with 2.4 × 107 molecules cm−3, twofold the measured C5H10O2 concentration as a conservative estimate. These bimolecular RO2 reaction steps together consume < 5% of the calculated HO-C5H8O2 radical concentration. It is to be noted that there is no experimental information on the alkoxy radical formation via RO2 + R′O2 → RO + R′O + O231 in our experiments, which is not considered yet in the HO-C5H8O2 radical balance. Assuming that alkoxy radical formation accounts for about half of the total product formation from RO2 radical self- and cross-reactions, i.e., a branching ratio of 0.5 as measured in the case of the HO-C2H4O2 self-reaction31, the experimentally observed and expected bimolecular RO2 reactions in total consume < 10% of the HO-C5H8O2 radicals for the highest isoprene conversion considered here. For lower isoprene conversion, and consequently lower HO-C5H8O2 radical concentrations, the bimolecular RO2 radical reactions are still less important.
Time-dependent product formation
The concentrations of HPALD, C5H8O4, and C4H8O5 increase in a parallel way among each other with rising reaction time qualitatively in accordance with the behavior of HPALD concentrations (and other isomerization products) as given by the work of Teng et al.13 (Fig. 7). Measured data for the HO-C5H8(O2)αO2 radicals with α = 0 and 1, which reveal an almost linear increase with time, are depicted in Supplementary Fig. 9. The linear increase of the HO-C5H8O2 signal with time confirms that bimolecular RO2 radical reactions, which become more important with rising RO2 concentrations, do not significantly influence the HO-C5H8O2 radical level. Our time-dependent HPALD concentrations are about twice the values by Teng et al.13, similar to the findings for changing isoprene conversion at a constant reaction time of 7.9 s (see Fig. 4c). The total amount of HPALD and “MW 116” reported by Teng et al.13 accounts for 80–90% of our HPALD concentrations, again similar to the behavior obtained from the measurement series with a reaction time of 7.9 s. The formation yields of C5H8O4 and C4H8O5 relative to HPALD from the present study are 0.20 ± 0.01 and 0.027 ± 0.005, respectively (Supplementary Fig. 10). Although the HPALD results of our study are about twice the data by Teng et al.13 (Fig. 7), our relative value for C5H8O4 is in reasonable agreement with [C5H8O4]/[HPALDs] = 0.14 given by Teng et al.13. There is no experimental information on C4H8O5 formation up to now in the literature.
Time-dependent measurement of closed-shell products. The red stars depict the signal measured at the (C5H8O3)H2NNH3+ mass, which is solely attributed to the HPALDs. Analysis was carried out using hydrazinium ionization. Reactant concentrations are [O3] = 1.04 × 1012, [TME] = 2.0 × 1011, and [isoprene] = 2.5 × 1012 molecules cm−3. The red dashed-dotted line shows the HPALD concentrations and the red dotted line the total amount of HPALD and “MW 116”, based on the work by Teng et al.13
1,6 H-shift product distribution
The predominant 1,6 H-shift products from Z-δ-HO-C5H8O2 radicals detected in the present study are the HPALDs accounting for > 75% of the closed-shell products from RO2 isomerization. This finding is different from the experimental results by Teng et al.13 who are stating a HPALD yield of 0.25 regarding the total 1,6 H-shift products. A possible reason for this discrepancy is speculative at the moment. However, it should be noted that these authors investigated a NOx system, and hydroperoxyacetone and hydroperoxyacetaldehyde, not visible in our experiment, were reported as additional 1,6 H-shift products.13 Moreover, also the reaction pathways leading to unknown “MW 116” products other than HPALDs, as observed by Teng et al.13, are unclear up to now. Table 1 compares the fraction of individual 1,6 H-shift products on the total 1,6 H-shift products of the work by Teng et al.13 with those from the present study. The data given by Teng et al.13 in Table 1 have been derived by a mass balance deviation. In contrast to that, the data from the present work arise from summing up all detected products.
On the absolute scale, total 1,6 H-shift product concentrations from our experiment amount to about 60% of the Teng et al.13 results (Fig. 8). The agreement is good taking into account an uncertainty of a factor of two in our case. Predicted total 1,6 H-shift products from the theoretical work by Peeters et al.12, however, are higher by a factor of about 7 compared with our findings.
Comparison of the total 1,6 H-shift product concentrations. Experimental results of the present study are depicted in red. Our signal measured at the product mass of C5H8O3 is solely attributed to the HPALDs. Error bars given for the total 1,6 H-shift products stand for the uncertainty by a factor of two for the lower limit concentrations. The blue full line shows the total 1,6 H-shift concentration based on the work by Teng et al.13 (Supplementary Note 2). The black full line shows the total 1,6 H-shift concentration based on the work by Peeters et al.12 (Supplementary Note 3)
The data given by Peeters et al.12 currently represent the base of the OH + isoprene subsystem of the Master Chemical Mechanism, MCM v3.3.1.41. Peeters et al.12 and Teng et al.13 use a different set of kinetic parameters for description of the RO2 radical processes resulting in different concentration profiles of the individual RO2 species and the 1,6 H-shift products, as exemplarily shown in Supplementary Figs 11 and 12 for our conditions. For clearly higher reaction times than in our experiment, t ≥ 50 s, calculated total 1,6 H-shift product concentrations are within a factor of two based on the data by Peeters et al.12 and Teng et al.13 (Supplementary Fig. 12). Expected total 1,6 H-shift product yield in forestlands with the highest isoprene emission and a bimolecular RO2 reactivity of about 0.02 s−1 is 0.28 using the data by Peeters et al.12 and 0.16 based on the work by Teng et al.13, both within a factor of 2 (Supplementary Fig. 13). The bimolecular RO2 reactivity of about 0.02 s−1 considers NO and HO2 radical concentrations of 5 × 108 and 1 × 109 molecules cm−3, respectively, as measured in the tropical forest42. For somewhat lower NO and HO2 radical levels and a supposed bimolecular RO2 reactivity of 0.005 s−1, the total 1,6 H-shift product yield reaches a value of up to 0.5, demonstrating the importance of RO2 isomerization steps for the first-generation products from OH + isoprene for pristine reaction conditions (Supplementary Fig. 13). The experiments of this study and the modeling calculations were conducted for a temperature of (297 ± 1) K. Higher temperatures are expected to significantly enhance the rate of RO2 radical isomerization steps, whereas bimolecular RO2 radical reactions are less temperature dependent43. Thus, the importance of 1,6 H-shift product formation is increasing with rising temperature and vice versa as shown for HPALD generation from a flow tube experiment19.
HPALD, C5H8O4, and C4H8O5 formation is connected with equal-molar HOx recycling being important for the HOx budget in isoprene-dominated forestlands42.
Discussion
Within the present work, direct observation of the first oxidation products from OH + isoprene with special attention to the RO2 isomerization products is reported. Reaction conditions were chosen in such a way that bimolecular RO2 reactions, with exception of NO + RO2 in a few runs, did not significantly influence the RO2 radical concentrations. The isomeric RO2 radicals HO-C5H8(O2)αO2 with α = 0, 1, and 2 were followed together with their closed-shell products HPALD, C5H8O4, and C4H8O5 formed via unimolecular pathways. The products, with the exception of the primarily formed HO-C5H8O2 radicals, are most likely measured with close to maximum sensitivity, i.e., analysis is approaching the “real” concentrations with an uncertainty by a factor of about two due to the expected uncertainty of the calculated calibration factor.
Furthermore, the accretion products C10H18O4 and C10H18O6, as well as C5H10O2, most likely HO-C5H8OH, have been detected from RO2 self- and cross-reactions. Products with the composition C5H10O3 were at least partly attributed to the isomeric hydroxy hydroperoxides HO-C5H8OOH.
Our results suggest that the formation of HO-C5H8(O2)2O2 radicals, and subsequently C4H8O5, is overvalued in the MCM v3.3.141. C4H8O5 represents only a minor product accounting only for 2–3% of the total closed-shell products formed via 1,6 H-shift of Z-δ-HO-C5H8O2 radicals. MCM v3.3.1. describes HPALD and C4H8O5 formation with the same yields for conditions of low bimolecular RO2 reactivity, as present in remote areas as well as in our experiment41. Apart from that, the formation of C5H8O4 as another important 1,6 H-shift product beside the HPALDs should be considered in atmospheric modeling. The present study also indicates that the formation of highly oxygenated molecules (HOMs) as the first-generation products from OH + isoprene is less important being qualitatively in line with a molar HOM yield of about 0.03% regarding the reacted isoprene obtained for similar reaction conditions as applied here44. A recently predicted molar HOM yield of up to 11% for conditions of a bimolecular RO2 reactivity of 0.01 s−145 is in contradiction to our experimental findings as well as to the low formation of secondary organic aerosol mass from the first-generation OH + isoprene products observed in smog chamber experiments46,47.
Methods
Flow system
Experiments have been performed in a free-jet flow system22,23,48 at a pressure of 1 bar of purified air and a temperature of (297 ± 1) K. Reaction times were in the range 3.0–7.9 s. The flow system consists of an outer tube (length: 200 cm, inner diameter: 16 cm) and a moveable inner tube (outer diameter: 9.5 mm) with a nozzle of 3 mm inner diameter. Ozone premixed with air (5 L min−1, STP) is injected through the inner tube into the main gas stream (95 L min−1 at standard temperature and pressure, STP), which contains the other reactants, TME and isoprene in most cases, diluted in air. The gas velocity at the nozzle outflow, nozzle: 15.9 m s−1, main flow: 0.13 m s−1, and the nozzle geometry ensure rapid reactant mixing. Effective reaction times between the nozzle outflow and the sampling point were experimentally determined by means of a “chemical clock”, i.e., by measuring ozone disappearance in the presence of excess of TME48.
OH radicals have been generated primarily via ozonolysis of TME29. Photolysis of isopropyl nitrite was used as an alternative OH radical source30. The photolysis was carried out downstream the mixing point of the gas streams by means of 8 NARVA 36 W Blacklight Blue lamps.
Reactant concentrations were in the range: [O3] = (1.2–230) × 1010, [TME] = 2.0 × 1011, [isoprene] = (5.0–25) × 1011, and [isoprene-1-13C] = 2.5 × 1012 molecules cm−3. Isopropyl nitrite concentrations in the photolysis experiments were (3.5–104) × 1010 molecules cm−3.
Product measurements
The detection of RO2 radicals and closed-shell products has been conducted by a CI-APi-TOF (chemical ionization–atmospheric pressure interface–time-of-flight) mass spectrometer (Airmodus, Tofwerk) that sampled from the center flow of the flow system with a rate of 10 L min−1 (STP). The resolving power of the mass spectrometer was > 3000 Th/Th. Ionization was carried out at atmospheric pressure using a Boulder-type inlet system49. Used reagent ions were protonated n-propyl-, ethyl- or methylamine, protonated hydrazine, acetate, or iodide. All products including the RO2 radicals, “prod”, were detected as a cluster with the reagent ion.
In addition, deprotonation products have been considered in the case of acetate. A comparison of the calculated and measured masses of product clusters, (prod)H2NNH3+, is given in Supplementary Table 3.
Reagent ions that consist of different protonated amines or hydrazine YH+ can cluster with ligands (X)m, where X stands for H2O or Y. (Product)reagent-ion cluster formation in reaction (5) proceeds at every collision if the ligand switch reaction is exothermic50. We calculated the formation enthalpies of (product) reagent-ion clusters (Fig. 3, Supplementary Table 1) revealing that YH+ forms strongly bound clusters with the products (e.g., (HPALD I)H2NNH3+ with 33.3 kcal mol−1). McNary and Armentroud51 recently investigated experimentally the bond energy of protonated hydrazine water clusters at 0 K and compared them with literature values of amine water clusters. According to that, H2NNH3+ is bound to water with 16.4 kcal mol−1 and CH3NH3+ with 17.8 kcal mol−1. We calculated the corresponding binding enthalpies for H2NNH3+, CH3NH3+, and n-C3H7NH3+ at 298 K with 19.2, 17.3, and 16.0 kcal mol−1, respectively, in good agreement with the range of bond energies obtained at 0 K51. Therefore, the ligand switch reaction (5) is exothermic and fast for all products of OH + isoprene including the RO2 radicals. The rather strong binding of the product ion clusters (prod)YH+ is important to prevent the clusters from being lost in collision-induced dissociation (CID) in the APi-TOF. In contrast, weakly bound clusters such as the reagent ions (X)mYH+ present in the CI region are lost by CID in the APi-TOF. That is the reason why (H2O)YH+ was not detected in the present experiments. In our previous study using (X)mNH4+ cluster ions only rather weak (H2O)NH4+ signals have been recorded in the NH4+-CI3-TOF instrument running at 80 mbar and using softest injection energies in the quadrupole ion transfer region36.
In the case of acetate and AcH ≡ acetic acid,
and for iodide,
possible ionization schemes have been discussed in the literature25,26,27,28.
Reagent ion formation
The reagent ions have been generated in a 35 L min−1 (STP) sheath flow of purified air or nitrogen (in the case of iodide) from an appropriate precursor compound after ionization with a 241Am source. Formed ions from the sheath flow were guided into the sample flow by an electric field without mixing of both gas streams.
In the case of ionization by acetate, a flow of 1–2 ml min−1 (STP) air over an acetic acid sample was added to the sheath flow forming the reagent ions (CH3COOH)mCH3COO−, m = 0, 1, 2.
In the case of iodide, tert.-butyl iodide premixed in a flask from a gas-metering unit was added to the sheath flow resulting in a tert.-butyl iodide concentration of 8 × 1010 molecules cm−3. The only detected reagent ion was I−. I3− was measureable in small traces.
Aminium reagent ions, protonated n-propyl-, ethyl-, or methylamine were generated from the corresponding amines using amine concentrations of (2.3–3.5) × 1011 molecules cm−3 in the sheath gas flow that had a relative humidity of about 1%. The amine samples were taken from a gas mixture in helium produced from a gas-metering unit. Tetrahydrofuran (THF) was added to the sheath flow with a concentration of 1 × 1013 molecules cm−3 in the case of methyl-aminium, to enhance the reagent-ion production. The enhancement is obviously caused by the proton transfer reaction of easily formed (THF)-H+ with CH3NH2 due to the higher proton affinity52. Detected reagent ions were the naked aminium ions, i.e., C3H7NH3+, C2H5NH3+, or CH3NH3+, with exception of the n-propyl aminium system where (C3H7NH2)C3H7NH3+ was visible to a lesser amount.
For the formation of hydrazinium ions, a flow of 1 ml min−1 (STP) air over a sample of hydrazine monohydrate, 64–65% N2H4, was added to the sheath flow that contained 1 × 1013 molecules cm−3 of THF for enhanced H2NNH3+ production. The only detectable reagent ion was H2NNH3+.
Determination of lower limit concentrations
RO2 radicals and closed-shell products, all termed in the following “prod”, were detected as clusters with the respective reagent ions, (prod)reagent-ion. Their concentrations were determined according to equation (8):
The quantities in equation (8) are the measured signal intensities. The “[reagent ion]” comprises the sum of the signal intensities of (CH3COOH)mCH3COO−, m = 0, 1, 2 in the case of acetate and (C3H7NH2)nC3H7NH3+, n = 0, 1 in the case of n-propyl-aminium. Duty cycle correction is applied to compensate for the mass-dependent transmission of the TOF mass spectrometer53,54. According to that the signal strengths were corrected with respect to the reagent ions at their nominal mass. For instance, in the case of acetate with respect to CH3COO− at nominal 59 Th are the duty cycle corrected counts per second of product i \({\mathrm{dcps}}\left( {\mathrm{i}} \right) = {\mathrm{cps}}\left( {\mathrm{i}} \right)\sqrt {\frac{{59}}{{{\mathrm{m}}_{\mathrm{i}}}}}\).
The lower limit value of the calibration factor f in equation (8) can be calculated considering the ion-molecule reaction in the CI-inlet, f = 1/(k × t × finlet)55,56, where k is the rate coefficient of the ion-molecule reaction, t the reaction time, and finlet considers the “prod” loss in the sampling tube. The rate coefficient k is set to (2–3) × 10−9 cm3 molecule−1 s−1, typical for ion-molecule reactions close to the collision limit57,58. Considering a 12% diffusion loss of “prod” in the short sampling tube (diffusion controlled wall loss for an assumed diffusion coefficient D = 0.08 cm2 s−1), finlet = 0.88, and a reaction time of the ion-molecule reaction t = 0.2–0.3 s, fcalc = (1.3–2.8) × 109 molecules cm−3 follows. The only reliable absolute calibration in our system at the moment is that used for sulfuric acid detection via H2SO4 + (HNO3)nNO3−, n = 0, 1, 2, 3, with a calibration factor fH2SO4,exp = 1.85 × 109 molecules cm−359. This value is in good agreement with the range of fcalc. The calibration factor f in equation (8) was set equal to fH2SO4,exp, to use a defined value and not a range. The uncertainty of the lower limit “prod” concentrations determined according to equation (8) is assumed to be not higher than a factor of two due to the expected uncertainty of the used calibration factor f, i.e., fH2SO4,exp = 1.85 × 109 compared with the range of fcalc = (1.3–2.8) × 109 molecules cm−3, and possible inaccuracy connected with the duty cycle correction.
Computational methods
The stability of the ion-molecule clusters, including the hydrated reagent-ion clusters, was modeled by calculating the formation of free energies and enthalpies of the clusters. The conformer sampling and computational methods were similar to those used by Hyttinen et al.60,61. The conformer sampling of the sample molecules and ion-molecule clusters was done using the Spartan ’14 program62. A systematic conformer sampling algorithm with Merck Molecular Force Field molecular mechanics optimization were used to obtain the initial sets of conformers. All conformers were then optimized at the B3LYP/6–31 + G* level of theory and ultrafine integration grid using Gaussian 0963. Final geometry optimizations and harmonic frequencies, also with ultrafine integration grid, were calculated for all conformers within 2 kcal mol−1 from the lowest electronic energy conformer at the ωB97X-D/aug-cc-pVTZ (aug-cc-pVTZ-PP for I) level of theory. Final single-point electronic energies were calculated at the DLPNO-CCSD(T)/def2-QZVPP level of theory using the corresponding auxiliary set and tight PNO settings, implemented in the Orca program64, version 4.0.0.2.
Data availability
All relevant data supporting the findings of this study are available within the article and the Supplementary Information, and from the corresponding author upon reasonable request.
Change history
22 February 2019
The original PDF and HTML versions of this Article contained two typographical errors, one in the Results section and one in the Discussion section. In both instances the word ‘undefined’ was erroneously inserted. This has been corrected in both the PDF and HTML versions of the Article.
References
Goldstein, A. H. & Galbally, I. E. Known and unexplored organic constituents in the Earth’s atmosphere. Environ. Sci. Technol. 41, 1514–1521 (2007).
Sindelarova, K. et al. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 14, 9317–9341 (2014).
Arneth, A. et al. Terrestrial biogeochemical feedbacks in the climate system. Nat. Geosci. 3, 525–532 (2010).
Seinfeld, J. H. & Pandis, S. N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change (Wiley, New York, 1998).
Zimmerman, P. R., Greenberg, J. P. & Westberg, C. E. Measurements of atmospheric hydrocarbons and biogenic emission fluxes in the Amazon boundary layer. J. Geophys. Res. 93, 1407–1416 (1988).
Fehsenfeld, F. et al. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Glob. Biogeochem. Cy. 6, 389–430 (1992).
Lee, S.-H. et al. Isoprene suppression of new particle formation: potential mechanisms and implications. J. Geophys. Res. Atmos. 121, 14621–14635 (2016).
Wennberg, P. O. et al. Gas-phase reactions of isoprene and its major oxidation products. Chem. Rev. 118, 3337–3390 (2018).
Jenkin, M. E. & Hayman, G. D. Kinetics of reactions of primary, secondary and tertiary β-hydroxy peroxyl radicals. J. Chem. Soc. Faraday Trans. 91, 1911–1922 (1995).
Morgan, C. A., Pilling, M. J., Tulloch, J. M., Ruiz, R. P. & Bayes, K. D. Direct determination of the equilibrium constant and thermodynamic parameters for the reaction C3H5 + O2 →← C3H5O2. J. Chem. Soc. Faraday Trans. 2 78, 1323–1330 (1982).
Peeters, J., Nguyen, T. L. & Vereecken, L. HOx radical regeneration in the oxidation of isoprene. Phys. Chem. Chem. Phys. 11, 5935–5939 (2009).
Peeters, J., Müller, J.-F., Stavrakou, T. & Nguyen, T. L. Hydroxyl radical recycling in isoprene oxidation driven by hydrogen bonding and hydrogen tunneling: the upgraded LIM1 mechanism. J. Phys. Chem. A 118, 8625–8643 (2014).
Teng, A. P., Crounse, J. D. & Wennberg, P. O. Isoprene peroxy radical dynamics. J. Am. Chem. Soc. 139, 5367–5377 (2017).
Jenkin, M. E., Boyd, A. A. & Lesclaux, R. Peroxy radical kinetics resulting from the OH-initiated oxidation of 1,3-butadiene, 2,3-dimethyl-1,3-butadiene and isoprene. J. Atmos. Chem. 29, 267–298 (1998).
Zhang, D., Zhang, R., Church, C. & North, S. W. Experimental study of hydroxyalkyl peroxy radicals from OH-initiated reactions of isoprene. Chem. Phys. Lett. 343, 49–54 (2001).
Medeiros, D. J., Blitz, M. A., James, L., Speak, T. H. & Seakins, P. W. Kinetics of the reaction of OH with isoprene over a wide range of temperature and pressure including direct observation of equilibrium with the OH adducts. J. Phys. Chem. A 122, 7239–7255 (2018).
Hermans, I., Müller, J. F., Nguyen, T. L., Jacobs, P. A. & Peeters, J. Kinetics of alpha-hydroxy-alkylperoxyl radicals in oxidation processes. HO2*-initiated oxidation of ketones/aldehydes near the tropopause. J. Phys. Chem. A 109, 4303–4311 (2005).
Crounse, J. D., Paulot, F., Kjaergaard, H. G. & Wennberg, P. O. Peroxy radical isomerization in the oxidation of isoprene. Phys. Chem. Chem. Phys. 13, 13607–13613 (2011).
Berndt, T. Formation of carbonyls and hydroperoxyenals (HPALDs) from the OH radical reaction of isoprene for low-NOx conditions: influence of temperature and water vapour content. J. Atmos. Chem. 69, 253–272 (2012).
Peeters, J. & Nguyen, T. L. Unusually fast 1,6-H shifts of enolic hydrogens in peroxy radicals: Formation of the first-generation C2 and C3 carbonyls in the oxidation of isoprene. J. Phys. Chem. A 116, 6134–6141 (2012).
Da Silva, G., Graham, C. & Wang, Z.-F. Unimolecular β-hydroxyperoxy radical decomposition with OH recycling in the photochemical oxidation of isoprene. Environ. Sci. Technol. 44, 250–256 (2010).
Berndt, T. et al. Hydroxyl radical-induced formation of highly oxidized organic compounds. Nat. Commun. 7, 13677 (2016).
Berndt, T. et al. Accretion product formation from self- and cross-reactions of RO2 radicals in the atmosphere. Angew. Chem. Int. Ed. 57, 3820–3824 (2018).
Berndt, T., Herrmann, H., Sipilä, M. & Kulmala, M. Highly oxidized second-generation products from the gas-phase reaction of OH radicals with isoprene. J. Phys. Chem. A 120, 10150–10159 (2016).
Bertram, T. H. et al. A field-deployable, chemical ionization time-of-flight mass spectrometer. Atmos. Meas. Tech. 4, 1471–1479 (2011).
Aljawhary, D., Lee, A. K. Y. & Abbatt, J. P. D. High-resolution chemical ionization mass spectrometry (ToF-CIMS): application to study SOA composition and processing. Atmos. Meas. Tech. 6, 3211–3224 (2013).
Huey, L. G., Hanson, D. R. & Howard, C. J. Reactions of SF6 − and I− with atmospheric trace gases. J. Phys. Chem. 99, 5001–5008 (1995).
Lee, B. H. et al. An iodide-adduct high-resolution time-of-flight chemical-ionization mass spectrometer: application to atmospheric inorganic and organic compounds. Environ. Sci. Technol. 48, 6309–6317 (2014).
Kroll, J. H., Sahay, S. R., Anderson, J. G., Demerjian, K. L. & Donahue, N. M. Mechanism of HOx formation in the gas-phase ozone-alkene reaction. 2. Prompt versus thermal dissociation of carbonyl oxides to form OH. J. Phys. Chem. A 105, 4446–4457 (2001).
Raff, J. D. & Finlayson-Pitts, B. J. Hydroxyl radical quantum yields from isopropyl nitrite photolysis in air. Environ. Sci. Technol. 44, 8150–8155 (2010).
Orlando, J. J. & Tyndall, G. S. Laboratory studies of organic peroxy radical chemistry: an overview with emphasis on recent issues of atmospheric significance. Chem. Soc. Rev. 41, 6294–6317 (2012).
Nozière, B. & Hanson, D. R. Speciated monitoring of gas-phase organic peroxy radicals by chemical ionization mass spectrometry: cross-reactions between CH3O2, CH3(CO)O2, (CH3)3CO2, and c-C6H11O2. J. Phys. Chem. A 121, 8453–8464 (2017).
Mackay, G. I. & Bohme, D. K. Proton-transfer reactions in nitromethane at 297K. Intern. J. Mass Spectrom. Ion Phys. 26, 327–343 (1978).
Viggiano, A. A., Seeley, J. V., Mundis, P. L., Williamson, J. S. & Morris, A. M. Rate constants for the reactions of XO3 −(H2O)n (X = C, HC, and N) and NO3 −(HNO3)n with H2SO4: Implications for atmospheric detection of H2SO4. J. Phys. Chem. A 101, 8275–8278 (1997).
Mackay, G. I., Tanner, S. D., Hopkinson, A. C. & Bohme, D. K. Gas-phase proton-transfer reactions of the hydronium ion at 298°K. Can. J. Chem. 57, 1518–1523 (1979).
Hansel, A., Scholz, W., Mentler, B., Fischer, L. & Berndt, T. Detection of RO2 radicals and other products from cyclohexene ozonolysis with NH4 + and acetate chemical ionization mass spectrometry. Atmos. Environ. 186, 248–255 (2018).
Malkin, T. L., Goddard, A., Heard, D. E. & Seakins, P. W. Measurements of OH and HO2 yields from the gas phase ozonolysis of isoprene. Atmos. Chem. Phys. 10, 1441–1459 (2010).
Berndt, T. & Böge, O. Formation of phenol and carbonyls from the atmospheric reaction of OH radicals with benzene. Phys. Chem. Chem. Phys. 8, 1205–1214 (2006).
Paulot, F. et al. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science 325, 730–733 (2009).
Ruppert, L. & Becker., K.-H. A product study of the OH radical-initiated oxidation of isoprene: formation of C5-unsaturated diols. Atmos. Environ. 34, 1529–1542 (2000).
Jenkin, M. E., Young, J. C. & Rickard, A. R. The MCMv3.3. 1 degradation scheme for isoprene. Atmos. Chem. Phys. 15, 11433–11459 (2015).
Lelieveld, J. et al. Atmospheric oxidation capacity sustained by a tropical forest. Nature 452, 737–740 (2008).
Praske, E. et al. Atmospheric autoxidation is increasingly important in urban and suburban North America. Proc. Natl Acad. Sci. USA 115, 64–69 (2018).
Jokinen, T. et al. Production of extremely low volatile organic compounds from biogenic emissions: Measured yields and atmospheric implications. Proc. Natl Acad. Sci. USA 112, 7123–7128 (2015).
Wang, S., Riva, M., Yan, C., Ehn, M. & Wang, L. Primary formation of highly oxidized multifunctional products in the OH-initiated oxidation of isoprene. A combined theoretical and experimental study. Environ. Sci. Technol. 52, 12255–12264 (2018).
Kroll, J. H., Ng, N. L., Murphy, S. M., Flagan, R. C. & Seinfeld, J. H. Secondary organic aerosol formation from isoprene photooxidation. Environ. Sci. Technol. 40, 1869–1877 (2006).
Ng, N. L. et al. Contribution of first- versus second-generation products to secondary organic aerosols formed in the oxidation of biogenic hydrocarbons. Environ. Sci. Technol. 40, 2283–2297 (2006).
Berndt, T. et al. Kinetics of the unimolecular reaction of CH2OO and the bimolecular reactions with the water monomer, acetaldehyde and acetone under atmospheric conditions. Phys. Chem. Chem. Phys. 17, 19862–19873 (2015).
Eisele, F. L. & Tanner, D. J. Ion-assisted tropospheric OH measurements. J. Geophys. Res. 96, 9295–9308 (1991).
Viggiano, A. A., Dale, F. & Paulson, J. F. Proton transfer reactions of H+(H2O)n=2-11 with methanol, ammonia, pyridine, acetonitrile, and acetone. J. Chem. Phys. 88, 2469–2477 (1989).
McNary, C. P. & Armentroud, P. B. Threshold collision-induced dissociation of protonated hydrazine and dimethylhydrazine clustered with water. J. Chem. Phys. 145, 214311 (2016).
Hunter, E. P. & Lias, S. G. Evaluated gas phase basicity and proton affinities of molecules: an update. J. Phys. Chem. Ref. Data 27, 413–656 (1998).
Breitenlechner, M. et al. PTR3: an instrument for studying the lifecycle of reactive organic carbon in the atmosphere. Anal. Chem. 89, 5824–5831 (2017).
Junninen, H. et al. A high-resolution mass spectrometer to measure atmospheric ion composition. Atmos. Meas. Tech. 3, 1039–1053 (2010).
Ehn, M. et al. A large source of low-volatility secondary organic aerosol. Nature 506, 476–479 (2014).
Berresheim, H., Elste, T., Plass-Dülmer, C., Eisele, F. L. & Tanner, D. J. Chemical ionization mass spectrometer for long-term measurements of atmospheric OH and H2SO4. Int. J. Mass Spectrom. 202, 91–109 (2000).
Viggiano, A. A., Seelev, J. V., Mundis, P. L., Williamson, J. S. & Morris, R. A. Rate constants for the reaction of XO3 −(H2O)n (X = C, HC, and N) and NO3 −(HNO3)n with H2SO4: Implications for atmospheric detection of H2SO4. J. Phys. Chem. A 101, 8275–8278 (1997).
Mackay, G. I. & Bohme, D. K. Proton-transfer reactions in nitromethane at 297 K. Intern. J. Mass Spectrom. Ion Phys. 26, 327–343 (1978).
Berndt, T. et al. H2SO4 formation from the gas-phase reaction of stabilized Criegee Intermediates with SO2: Influence of water vapour content and temperature. Atmos. Environ. 89, 603–612 (2014).
Hyttinen, N., Rissanen, M. P. & Kurtén, T. Computational comparison of acetate and nitrate chemical ionization of highly oxidized cyclohexene ozonolysis intermediates and products. J. Phys. Chem. A 120, 2172–2179 (2017).
Hyttinen, N. et al. Computational comparison of different reagent ions in the chemical ionization of oxidized multifunctional compounds. J. Phys. Chem. A 121, 269–279 (2018).
Wavefunction Inc.: Spartan’14, Irvine, CA (2014).
Frisch, M. J. et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT (2009).
Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 73–78 (2012).
Acknowledgements
We thank J. Crounse and T. Kurtén for helpful discussions, K. Pielok and A. Rohmer for technical assistance, and the tofTools team for providing the data analysis toolbox. N.H. thanks the Academy of Finland for funding and CSC–IT Center for Science, Finland, for computational resources.
Author information
Authors and Affiliations
Contributions
T.B. designed and conducted the experiments, did the data analysis, and wrote the manuscript. N.H. did the calculations on the stability of the ion-molecule clusters. H.H. contributed to the data interpretation. A.H. supported the development of the mass spectrometric techniques. All authors have discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berndt, T., Hyttinen, N., Herrmann, H. et al. First oxidation products from the reaction of hydroxyl radicals with isoprene for pristine environmental conditions. Commun Chem 2, 21 (2019). https://doi.org/10.1038/s42004-019-0120-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-019-0120-9
This article is cited by
-
Direct sulfuric acid formation from the gas-phase oxidation of reduced-sulfur compounds
Nature Communications (2023)
-
Efficient alkane oxidation under combustion engine and atmospheric conditions
Communications Chemistry (2021)
-
Quantum chemical study of gas-phase reactions of isoprene with OH radicals producing highly oxidised second-generation products
Journal of Molecular Modeling (2021)
-
Rapid conversion of isoprene photooxidation products in terrestrial plants
Communications Earth & Environment (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.