Abstract
Combining endurance training with resistance training (RT) may attenuate skeletal muscle hypertrophic adaptation versus RT alone; however, the underlying mechanisms are unclear. We investigated changes in markers of ribosome biogenesis, a process linked with skeletal muscle hypertrophy, following concurrent training versus RT alone. Twenty-three males underwent eight weeks of RT, either performed alone (RT group, n = 8), or combined with either high-intensity interval training (HIT+RT group, n = 8), or moderate-intensity continuous training (MICT+RT group, n = 7). Muscle samples (vastus lateralis) were obtained before training, and immediately before, 1 h and 3 h after the final training session. Training-induced changes in basal expression of the 45S ribosomal RNA (rRNA) precursor (45S pre-rRNA), and 5.8S and 28S mature rRNAs, were greater with concurrent training versus RT. However, during the final training session, RT further increased both mTORC1 (p70S6K1 and rps6 phosphorylation) and 45S pre-rRNA transcription-related signalling (TIF-1A and UBF phosphorylation) versus concurrent training. These data suggest that when performed in a training-accustomed state, RT induces further increases mTORC1 and ribosome biogenesis-related signalling in human skeletal muscle versus concurrent training; however, changes in ribosome biogenesis markers were more favourable following a period of short-term concurrent training versus RT performed alone.
Similar content being viewed by others
Introduction
Simultaneously incorporating both resistance and endurance training into a periodised training program, termed concurrent training1, can attenuate resistance training adaptations such as muscle hypertrophy, compared with resistance training performed alone2,3,4. This effect is potentially mediated by an altered balance between post-exercise skeletal muscle protein synthesis (MPS) and breakdown, subsequently attenuating lean mass accretion. The mechanistic target of rapamycin complex 1 (mTORC1) is a key mediator of load-induced increases in MPS and subsequently muscle hypertrophy5,6. The activity of mTORC1 is antagonised by activation of the 5′ adenosine monophosphate-activated protein kinase (AMPK), which acts to restore perturbations in cellular energy balance by inhibiting anabolic cellular processes and stimulating catabolism7. For example, in rodent skeletal muscle, low-frequency electrical stimulation mimicking endurance exercise-like contractions promotes AMPK activation and inhibition of mTORC1 signalling8.
Subsequent work in humans9,10,11,12,13,14,15,16,17,18 has focused on the hypothesis that attenuated muscle hypertrophy with concurrent training2,4,19 may be explained by AMPK-mediated inhibition of the mTORC1 pathway. Several studies, however, have demonstrated that single sessions of concurrent exercise do not compromise either mTORC1 signalling or rates of MPS9,10,16,17,18, and may even potentiate these responses14, compared with resistance exercise performed alone. However, a limitation of these studies is that most have examined these responses in either untrained individuals16,17,18 or those who are relatively unaccustomed to the exercise protocol14,20. Given short-term training increases the mode-specificity of post-exercise molecular responses21,22, examining perturbations to molecular signalling and gene expression in relatively training-unaccustomed individuals may confound any insight into the potential molecular mechanisms responsible for interference following concurrent training23.
Transient changes in translational efficiency (i.e., rates of protein synthesis per ribosome) after single sessions of concurrent exercise, as indexed by skeletal muscle mTORC1 signalling or rates of MPS, in relatively training-unaccustomed individuals therefore do not appear to explain interference to muscle hypertrophy following longer-term concurrent training. However, rates of cellular protein synthesis are determined not only by transient changes in translational efficiency, but also by cellular translational capacity (i.e., amount of translational machinery per unit of tissue, including ribosomal content)24. Ribosomes are supramolecular ribonucleoprotein complexes functioning at the heart of the translational machinery to convert mRNA transcripts into protein24, and ribosomal content dictates the upper limit of cellular protein synthesis25. Early rises in protein synthesis in response to anabolic stimuli (e.g., a single bout of resistance exercise) are generally thought to be mediated by transient activation of existing translational machinery, whereas prolonged anabolic stimuli (e.g., weeks to months of RE training) induces an increase in total translational capacity via ribosome biogenesis24.
Ribosome biogenesis is a complex, well-orchestrated process involving transcription of the polycistrionic 45S rRNA (ribosomal RNA) precursor (45S pre-rRNA), processing of the 45S pre-rRNA into several smaller rRNAs (18S, 5.8S and 28S rRNAs), assembly of these rRNAs and other ribosomal proteins into ribosomal subunits (40S and 60S), and nuclear export of these ribosomal subunits into the cytoplasm24,26. The synthesis of the key components of the ribosomal subunits is achieved via the coordinated actions of three RNA polymerases (RNA Pol-I, -II, and -III). The RNA Pol-I is responsible for the transcription of the 45S pre-rRNA in the nucleolus, which is considered the rate-limiting step in ribosome biogenesis27. The 45S pre-rRNA is subsequently cleaved into the 18S, 5.8S and 28S rRNAs, which undergo post-transcriptional modifications via interactions with small nuclear ribonucleoproteins and several protein processing factors. The RNA Pol-II is responsible for the transcription of ribosomal protein-encoding genes, whereas RNA Pol-III mediates the nucleoplasmic transcription of 5S rRNA and tRNAs (transfer RNAs)26.
As well as controlling translational efficiency, the mTORC1 is a key mediator of ribosome biogenesis by regulating transcription factors for genes encoding RNA Pol-I (see Fig. 1) and -III25. The transcription of rDNA by RNA Pol-I requires the transcription factor SL-1 (selectivity factor-1), a component of which is TIF-1A (transcription initiation factor 1A; also known as RRN5), as well as other regulatory factors including POLR1B (polymerase [RNA] 1 polypeptide B). Inhibition of mTORC1 by rapamycin inactivates TIF-1A, which impairs the transcription of the 45S pre-rRNA by RNA Pol-I28. Inhibition of mTORC1 also inactivates UBF (upstream binding factor)29, a transcription factor also associated with SL-1, while the key mTORC1 substrate p70S6K1 promotes UBF activation and RNA Pol-I-mediated rDNA transcription29. As well as regulation by mTORC1 signalling, the cyclins (including cyclin-D1) and cyclin-dependent kinases (CDKs) can also regulate UBF via phosphorylation on Ser388 and Ser484, which are required for UBF activity30,31. In addition to regulation of RNA Pol-1, mTORC1 also associates with a number of RNA Pol-III genes that synthesise 5 S rRNA and tRNA32.
Overview of the role of mTORC1 signalling in promoting ribosome biogenesis following a single session of resistance exercise, and the potential effect of incorporating endurance training (i.e., performing concurrent training). Adapted with permission from24. Ribosome biogenesis involves transcription of the 45S rRNA (ribosomal RNA) precursor (45S pre-rRNA) (A) mediated by RNA Polymerase I (Pol-I), processing of the 45S pre-rRNA into several smaller rRNAs (18S, 5.8S and 28S rRNAs) (B), assembly of these rRNAs and other ribosomal proteins into ribosomal subunits (40S and 60S) (C), and nuclear export of these ribosomal subunits into the cytoplasm24,26 (D). As well as regulating translational efficiency via downstream control of p70S6K (p70 kDa ribosomal protein subunit kinase 1) and 4E-BP1 (eukaryotic initiation factor 4E binding protein 1) (E), mTORC1 is a key mediator of ribosome biogenesis by regulating transcription factors for genes encoding RNA Pol-I (and also RNA Pol-II and –III, which are not shown in figure)25. Transcription of the 45S pre-rRNA by RNA Pol-I requires a transcriptional complex including TIF-1A (transcription initiation factor 1A; also known as RRN5) and UBF (upstream binding factor), both of which are regulated by the mTORC1 pathway28,29 (F). Activation of AMPK is known to inhibit mTORC1 signalling in rodent skeletal muscle64, and AMPK activation in skeletal muscle is traditionally associated with endurance-type exercise. However, whether signalling events initiated by endurance training, when performed concurrently with resistance training, have the potential to interfere with mTORC1-mediated regulation of ribosome biogenesis is currently unclear (G).
Studies in both human33,34,35 and rodent skeletal muscle36,37,38,39,40,41 suggest ribosome biogenesis, as indexed by increases in total RNA content (>85% of which comprises rRNA)24, and increased mRNA expression of several RNA Pol-I regulatory factors, including UBF, cyclin D1 and TIF-1A, occurs concomitantly with muscle hypertrophy. In addition, attenuated rodent skeletal muscle hypertrophy with ageing35,42,43 and rapamycin treatment40 is associated with reduced markers of ribosome biogenesis, suggesting translational capacity is closely linked to the regulation of skeletal muscle mass. Despite the links between skeletal muscle hypertrophy and ribosome biogenesis24,33,34, studies investigating molecular interference following concurrent exercise in human skeletal muscle have only measured transient (<6 h) post-exercise changes in translational efficiency (as indexed by mTORC1 signalling) and MPS9,10,11,12,13,14,15,16,17,18. No studies have investigated changes in markers of ribosome biogenesis either after single bouts of concurrent exercise or following periods of concurrent training. Whether attenuated muscle hypertrophy following concurrent training could be explained, at least in part, by attenuated ribosome biogenesis is unknown.
The aim of this study was therefore to investigate changes in markers of ribosome biogenesis and mTORC1 signalling after eight weeks of concurrent training compared with resistance training undertaken alone. A secondary aim was to determine the potential role of endurance training intensity in modulating skeletal muscle ribosome biogenesis adaptation to concurrent training, by comparing concurrent training incorporating either high-intensity interval training (HIT) or moderate-intensity continuous training (MICT). The induction of these responses in skeletal muscle was also investigated following a single exercise session performed post-training. It was hypothesised that compared with resistance training alone, concurrent training would attenuate the training-induced increase in markers of skeletal muscle ribosome biogenesis, and the induction of mTORC1 signalling, both at rest post-training and after a single training session performed in a training-accustomed state. It was further hypothesised that concurrent training incorporating HIT would preferentially attenuate training-induced skeletal muscle hypertrophy relative to resistance training alone, and this would be associated with an attenuation of markers of skeletal muscle ribosome biogenesis.
Results
For a detailed summary of statistical data for all within- and between-group effects that were considered substantial in magnitude and/or statistically significant (P < 0.05), see Tables 1 and 2, respectively. A full list of all within- and between-group statistical comparisons are shown in Supplementary data Tables 1 and 2, respectively.
Training-induced changes in maximal strength and lean body mass
In brief, and as previously reported44, one-repetition maximum (1-RM) leg press strength was improved from pre- to post-training for all training groups (see Table 1). Consistent with previous reports of interference to strength development with concurrent training3,19, the magnitude of this change was greater for RT vs. both HIT+RT and MICT+RT (see Table 2). Despite the differences in strength, lower-body lean mass was similarly increased for RT and MICT+RT; however, this increase was attenuated for HIT+RT (see Supplementary data Table 1).
Physiological and psychological responses to the final training session
During the final training session, there was a higher average heart rate (mean difference range ± 90% confidence limits, 14 ± 12 to 19 ± 14 bpm; ES range ± 90% confidence limits, 1.04 ± 0.88 to 1.22 ± 0.89; P ≤ 0.043; Table 3) and rating of perceived exertion (RPE) (2 ± 2 to 4 ± 2 AU; ES, 1.51 ± 0.86 to 2.15 ± 0.87; P ≤ 0.06) for HIT compared with MICT. During the final training session, venous blood lactate (Table 3) was also higher for HIT compared with MICT at all time points both during cycling (mean difference range, 0.8 ± 0.5 to 4.5 ± 1.1 mmol·L−1; ES range, 1.46 ± 0.87 to 3.65 ± 0.85; P ≤ 0.01) and during the 15-min recovery period after cycling (3.5 ± 1.0 to 5.0 ± 1.2 mmol·L−1; ES, 3.11 ± 0.85 to 3.68 ± 0.85; P < 0.001). Venous blood glucose (Table 3) was also higher for HIT vs. MICT after 16, 22, 28 and 34 min cycling (0.4 ± 0.7 to 1.6 ± 0.9 mmol·L−1; ES, 0.54 ± 0.86 to 1.52 ± 0.86; P ≤ 0.039), and during the 15-min recovery period after cycling (0.9 ± 0.7 to 1.8 ± 1.0 mmol·L−1; ES, 1.11 ± 0.85 to 1.50 ± 0.85; P ≤ 0.041).
After completion of RE in the final training session, venous blood lactate (Table 4) was higher for HIT+RT vs. RT after 0, 2, 5, 10, 60, 90 and 180 min of recovery (0.1 ± 0.1 to 1.4 ± 0.9 mmol·L−1; ES, 0.80 ± 0.84 to 1.74 ± 0.84; P ≤ 0.095), and higher for HIT+RT vs. MICT+RT at all timepoints (0.1 ± 0.1 to 1.1 ± 1.4 mmol·L−1; ES, 0.73 ± 0.87 to 1.82 ± 0.86; P ≤ 0.161). Post-RE venous blood glucose (Table 4) was lower for HIT+RT vs. RT after 2, 10, and 30 min of recovery (0.3 ± 0.2 to 0.3 ± 0.3 mmol·L−1; ES, −0.65 ± 0.84 to −1.02 ± 0.84; P ≤ 0.193), and higher for HIT+RT vs. RT after 60 min of recovery (0.4 ± 0.4 mmol·L−1; ES, 0.88 ± 0.84; P = 0.077). Blood glucose was higher for MICT vs. HIT+RT at +30 min of recovery (0.3 ± 0.2 mmol·L−1; ES, 1.29 ± 0.86; P = 0.021), and lower for HIT+RT vs. MICT+RT at +60 min of recovery (0.2 ± 0.2 mmol·L−1; ES, −1.09 ± 0.85; P = 0.045).
Training-induced changes in markers of ribosome biogenesis
The total RNA content of skeletal muscle was measured as an index of translational capacity (and hence ribosomal content), since ribosomal RNA comprises over 85% of the total RNA pool45. Pre-training total RNA content was higher for the RT group vs. both HIT+RT (38 ± 17%; ES, −1.48 ± 0.84; P = 0.005; Table 5) and MICT+RT (25 ± 12%; ES, 1.47 ± 0.85; P = 0.010). Total RNA content was decreased in the basal state post-training in the RT group (see Table 1), and was not substantially changed for either HIT+RT or MICT+RT (see Supplementary data Table 1). The change in total RNA content between pre- and post-training was, however, greater for both HIT+RT and MICT+RT vs. RT (see Table 2).
Given the observed changes in skeletal muscle RNA content, we also investigated training-induced changes in components of the ribosomal machinery in skeletal muscle, including expression levels of the 45S rRNA precursor, and the mature forms of the 5.8S, 18S and 28S rRNAs.
Expression of 45S pre-rRNA was unaltered by training for all groups (Fig. 2); however, greater training-induced changes in 45S pre-rRNA expression were noted for both HIT+RT and MICT+RT vs. RT (see Table 2). There were no substantial changes, nor between-group differences, in 45S pre-rRNA expression during the final training session for either training group.
Expression of 45S pre-rRNA relative to the geometric mean of the expression of three housekeeping genes (HKG) (cyclophillin, β2M and TBP) before (PRE-T) and after (POST-T) eight weeks of either RT alone, or RT combined with either high-intensity interval training (HIT+RT) or moderate-intensity continuous training (MICT+RT), and 1 h and 3 h after a single exercise bout performed post-training. Data presented are means ± SD and expressed relative to the PRE-T value for each corresponding group. *P < 0.05 vs. PRE-T, achange between PRE-T and POST-T substantially different vs. RT.
We used specifically-designed primers34 to distinguish between the expression levels of mature rRNA species [designated as 5.8S, 18S and 28S (mature) rRNAs] and those transcripts still bound to the 45S rRNA precursor and hence indicative only of changes in 45S pre-rRNA expression [designated as 5.8S, 18S and 28S (span) rRNAs].
Expression of 5.8S rRNA (mature) was lower post-training for RT (see Table 1; Fig. 3A). Both HIT+RT and MICT+RT induced greater post-training increases in 5.8S rRNA (mature) expression vs. RT (see Table 2). Neither training group induced substantial post-exercise changes in 5.8S rRNA (mature) expression during the final training session. Expression of 5.8S rRNA (span) was also lower post-training for RT (see Table 1; Fig. 3B), and the basal training-induced change in 5.8S rRNA (span) expression was greater for HIT+RT vs. RT (see Table 2).
Expression of the mature rRNA transcripts 5.8S rRNA (A), 18S rRNA (C), and 28S rRNA (E), and rRNA transcripts bound to the 45S pre-RNA precursor: 5.8S rRNA (span) (B) 18S rRNA (span) (D) and 28S rRNA (span) (F) relative to the geometric mean of the expression of three housekeeping genes (HKG) (cyclophillin, β2M and TBP) before (PRE-T) and after (POST-T) eight weeks of either RT alone, or RT combined with either high-intensity interval training (HIT+RT) or moderate-intensity continuous training (MICT+RT), and 1 h and 3 h after a single exercise bout performed post-training. Data presented are means ± SD and expressed relative to the PRE-T value for each corresponding group. *P < 0.05 vs. PRE-T, †P < 0.05 vs. POST-T, achange between PRE-T and POST-T substantially greater vs RT.
Expression of 18S rRNA (mature) was not substantially different at any time point, nor were there any substantial between-group differences in changes in 18S rRNA (mature) expression (Fig. 3C). There were also no substantial effects of training or any between-group differences in changes in 18S rRNA (span) expression (Fig. 3D), although increased 18S rRNA (span) expression was noted at +3 h during the final training bout for MICT+RT (see Table 1; Fig. 3D).
Resting levels of 28S rRNA (mature) expression were reduced post-training for RT (see Table 1; Fig. 3E). Greater training-induced changes in basal 28S rRNA expression were noted for both HIT+RT and MICT+RT vs. RT (see Table 2). Neither training group induced substantial post-exercise changes in 28S rRNA expression during the final training session. No changes in resting 28S rRNA (span) expression were noted in response to training for either group (Fig. 3F). However, HIT+RT induced greater training-induced changes in basal 28S rRNA (span) expression compared with RT (see Table 2).
Ribosome biogenesis-related signalling responses
To determine potential upstream molecular events associated with changes in markers of ribosome biogenesis with concurrent versus single-mode resistance training, we investigated the regulation of key proteins (TIF-1A, UBF and cyclin D1) involved in promoting 45S rRNA precursor expression (Fig. 1).
Post-training, basal levels of TIF-1ASer649 phosphorylation were increased only for HIT+RT (see Table 1; Fig. 4A). During the final training session, only RT was sufficient to increase TIF-1A phosphorylation at both +1 h and +93 h (see Table 1), and this increase (between POST-T to +3 h) was greater than for both HIT+RT and MICT+RT (see Table 2).
Phosphorylation of TIF-1ASer649 (A), UBFSer388 (B), and total protein content of cyclin D1 (C) before (PRE-T) and after (POST-T) eight weeks of either RT alone, or RT combined with either high-intensity interval training (HIT+RT) or moderate-intensity continuous training (MICT+RT), and 1 h and 3 h after a single exercise bout performed post-training. Data presented are means ± SD and expressed relative to the PRE-T value for each corresponding group. *P < 0.05 vs. PRE-T, †P < 0.05 vs. POST-T. Change from POST-T substantially greater vs. eHIT+RT, fMICT+RT.
A similar pattern was observed for UBFSer388 phosphorylation, although no training group showed altered UBFSer388 phosphorylation in the basal state post-training (see Fig. 4B). As observed with TIF-1A, only RT was sufficient to increase UBF phosphorylation during the final training session at both +1 h and +3 h (see Table 1). RT also induced greater changes in UBF phosphorylation during the final training session at both +1 h and +3 h vs. both HIT+RT and MICT+RT (see Table 2).
As observed for UBF, the protein content of cyclin D1 was unchanged between pre- and post-training for all training groups (Fig. 4C). However, a post-exercise reduction in cyclin D1 protein content was noted for HIT+RT at +1 h during the final training session (see Table 1).
mRNA responses related to ribosome biogenesis
We also measured the mRNA levels of select genes (TIF-1A, UBF, POLR1B, and cyclin D1) involved in promoting 45S rRNA precursor expression (see Fig. 1).
Neither training group altered basal TIF-1A mRNA content post-training (Fig. 5A). During the final training session, only RT and MICT+RT increased TIF-1A mRNA expression at +3 h (see Table 1).
mRNA expression of TIF-1A (A), UBF (B), POLR1B (C), and cyclin D1 (D) relative to the geometric mean of the expression of three housekeeping genes (HKG) (cyclophillin, β2M and TBP) before (PRE-T) and after (POST-T) eight weeks of either RT alone, or RT combined with either high-intensity interval training (HIT+RT) or moderate-intensity continuous training (MICT+RT), and 1 h and 3 h after a single exercise bout performed post-training. Data presented are means ± SD and expressed relative to the PRE value for each corresponding group. *P < 0.05 vs. PRE-T, †P < 0.05 vs. POST-T. Change from POST-T substantially greater vs. d = RT.
Basal levels of UBF mRNA post-training were not altered by either training group (Fig. 5B). There were no substantial changes in UBF expression during the final training session for either training group.
Basal expression of POLR1B mRNA was reduced post-training by RT alone (see Table 1; Fig. 5C). Only HIT+RT and MICT+RT increased POLR1B mRNA expression at +3 during the final training session (see Table 1).
Post-training basal expression of cyclin D1 mRNA was increased only for HIT+RT (see Table 1; Fig. 5D). No post-exercise changes in cyclin D1 mRNA were noted in response to the final training session for either training group.
AMPK/mTORC1-related signalling responses
Given the observed changes in TIF-1A and UBF phosphorylation following training, we investigated changes in mTORC1 signalling as a potential upstream regulator of these responses25, as well as AMPK signalling as a negative regulator of mTORC1 signalling7.
The phosphorylation of AMPKThr172 was unchanged in the basal state post-training for all groups (Fig. 6A). AMPK phosphorylation was, however, increased by RT at +1 h during the final training session (see Table 1). RT also induced a greater change in AMPK phosphorylation at +3 h during the final training session vs. MICT+RT (see Table 2), but not vs. HIT+RT (see Supplementary Data Table 2).
Phosphorylation of AMPKThr172 (A), ACCSer79 (B), mTORSer2448 (C), p70S6KThr389 (D), rps6Ser235/236 (E) and 4E-BP1Thr36/47 (F) before (PRE-T) and after (POST-T) eight weeks of either RT alone, or RT combined with either high-intensity interval training (HIT+RT) or moderate-intensity continuous training (MICT+RT), and 1 h and 3 h after a single exercise bout performed post-training. Data presented are means ± SD and expressed relative to the PRE value for each corresponding group. *P < 0.05 vs. PRE-T, †P < 0.05 vs. POST-T. Change from POST-T substantially greater vs. eHIT+RT, fMICT+RT.
As observed with AMPKThr172 phosphorylation, neither training group had altered ACCSer79 phosphorylation in the basal state post-training (Fig. 6B). However, reductions in ACC phosphorylation were noted at +1 h in the final training session for both RT and MICT+RT, and at +3 h for RT (see Table 1). Compared with RT, HIT+RT induced greater changes in ACC phosphorylation during the final training session at both +1 h and +3 h (see Table 2).
As observed with both AMPKThr172 and ACCSer79, the phosphorylation of mTORSer2448 was unchanged in the basal state post-training for all training groups (Fig. 6C). In response to the final training bout, mTOR phosphorylation was increased at +1 h only for RT (see Table 1), and not for either HIT+RT or MICT+RT (see Supplementary Data Table 1), and was increased at +3 h only for HIT+RT (see Table 1).
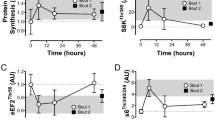
The observed changes in p70S6K1Thr389 phosphorylation mirrored changes in mTORSer2448 phosphorylation (Fig. 6D). Indeed, only RT was sufficient to increase p70S6K1Thr389 phosphorylation at +1 h during the final training session (see Table 1). RT alone also induced a greater change in p70S6K1 phosphorylation at +3 h compared with both HIT+RT and MICT+RT (see Table 2).
All training groups increased rps6Ser235/236 phosphorylation during the final training bout at +1 h and +3 h (see Table 1; Fig. 6E). The change in rps6 phosphorylation at +3 h was, however, greater for RT vs. MICT+RT (see Table 2) but not vs. HIT+RT (see Supplementary Data Table 2).
Despite the evidence of increased mTORC1 signalling as indexed via enhanced p70S6K1Thr389 phosphorylation, we observed no between-group differences in 4E-BP1Thr36/47 phosphorylation at any time point (Fig. 6F).
Muscle fibre CSA responses
We also performed immunohistochemical analyses to determine fibre-type specific changes in muscle fibre CSA induced by the concurrent versus single-mode resistance training protocols (see Table 5).
Type I muscle fibre CSA was increased by RT alone (see Table 1), but not for either HIT+RT or MICT+RT (see Supplementary Data Table 1). The training-induced change in type I fibre CSA was also greater for RT compared with HIT+RT, but not vs. MICT+RT (see Table 2).
Type II muscle fibre CSA was not substantially altered by either training group (see Supplementary Data Table 1). Representative immunohistochemical images are shown in Fig. 7.
Representative immunohistochemical images of muscle cross-sections obtained before (PRE) and after (POST) eight weeks of either RT alone (images (A) and (B), respectively), or RT combined with either high-intensity interval training (HIT+RT; images (C) and (D), respectively) or moderate-intensity continuous training (MICT+RT; images (E and F) respectively). Muscle fibre membranes are stained red, type I muscle fibres are stained green, and type II muscle fibres are unstained.
Discussion
Previous investigations on molecular responses and adaptations in skeletal muscle to concurrent training have focused almost exclusively on markers of post-exercise translational efficiency (i.e., mTORC1 signalling and rates of MPS)9,10,11,12,13,14,15,16,17,18. For the first time, we present data on the regulation of translational capacity (i.e., ribosome biogenesis) in skeletal muscle with concurrent training compared with resistance training performed alone. The major findings were that training-induced changes in markers of ribosome biogenesis, including total RNA content and expression of some mature rRNA species (i.e., 5.8S and 28S, but not 18S) were more favourable following concurrent training compared with resistance training alone, and irrespective of the endurance training intensity employed. These responses occurred despite a single bout of resistance exercise, when performed post-training, further inducing both mTORC1- and ribosome biogenesis-related signalling (i.e., TIF-1 and UBF phosphorylation) compared with concurrent exercise. These observations also contrasted with our findings regarding changes in muscle fibre-type specific hypertrophy, which was greater in type I muscle fibres for the resistance training group, suggesting a disconnect between training-induced changes in markers of ribosome biogenesis and muscle fibre hypertrophy.
To investigate the effects of concurrent versus single-mode resistance training on markers of skeletal muscle ribosome biogenesis, we measured training-induced changes in total RNA content and basal expression of mature ribosome species 5.8S, 18S, and 28S, as well as early post-exercise changes in mature rRNA expression. Contrary to our hypothesis, resistance training alone induced small decreases in the levels of both the 5.8S and 28S rRNAs in the basal state post-training, while the training-induced change in both of these mature rRNA species was greater with concurrent exercise compared with resistance training alone. Neither training protocol induced any changes in 18S rRNA expression. Previous work in humans has observed basal increases in 5.8S, 18S, and 28S rRNA expression in human skeletal muscle after 8 weeks of resistance training, all of which were reduced 1 h following a single session of resistance exercise performed post-training34. The present data contrast with these findings by suggesting that resistance training performed alone was an insufficient stimulus to increase mature rRNA content, whereas concurrent exercise was sufficient to increase mature 5.8S and 28S expression after a single post-training exercise bout.
Consistent with the training-induced changes in both 5.8S and 28S rRNA expression with resistance training performed alone, a small reduction in basal total RNA content in skeletal muscle was observed within this cohort. Despite this paradoxical finding, it is interesting to note total RNA content was higher at pre-training for the RT group compared with both the HIT+RT and MICT groups (1.6- and 1.3-fold, respectively). The reason for this between-group discrepancy at baseline is not immediately clear, given we previously showed no differences in baseline lean mass measured via DXA or lower-body 1-RM strength in these participants44, suggesting other factors may have influenced the between-group differences in baseline skeletal muscle RNA content. It is also possible that the training program provided an insufficient stimulus to at least maintain this elevated basal RNA content for the RT group. Studies demonstrating robust increases in total RNA content concomitantly with rodent skeletal muscle hypertrophy typically employ supraphysiological methods for inducing muscle hypertrophy, such as synergist ablation36,39,46,47, a stimulus that is clearly not replicated by resistance training in human models. Participant training status may also impact upon training-induced changes in ribosome biogenesis in humans. The participants in the present study were actively engaging in resistance and/or endurance training for at least 1 year prior to commencing the study, suggesting a higher training status compared with those of Figueiredo et al.34 who were likely untrained (although this was not made explicitly clear) and asked to refrain from resistance training for 3 weeks prior to the study34. It is also possible that between-group differences in training volume, which was clearly higher for the concurrent training groups compared with the RT group, may have impacted upon the training-induced changes in total skeletal muscle RNA content.
Despite the observed changes in skeletal muscle RNA content, resistance training alone was sufficient to increase type I, but not type II, muscle fibre CSA. The lack of any substantial type II fibre hypertrophy is likely due, at least in part, to the specific nature of the resistance training program employed, which was perhaps better-oriented for enhancing maximal strength rather than lean mass44. Indeed, previously-published data indicates that the resistance training protocol employed in the present study was effective in improving maximal strength and measures of lean mass44, although these changes did not transfer to detectable type II fibre hypertrophy. Nevertheless, in agreement with previous research2,4, the training-induced increase in type I muscle fibre CSA was attenuated with concurrent exercise, albeit only when incorporating HIT, compared with resistance training performed alone. Despite these between-group differences in fibre-type specific hypertrophy, we could find no evidence that the training-induced changes in lean mass or muscle fibre CSA were correlated with changes in total RNA content of skeletal muscle (data not shown). The apparent disconnect between training-induced changes in total RNA content and markers of muscle hypertrophy, both at the whole-body and muscle-fibre levels, suggests further investigation is required into relationship between changes in translational capacity and resistance training-induced hypertrophy in human skeletal muscle, particularly in the context of concurrent training.
To circumvent the potentially confounding influence of training status on the mode-specificity of post-exercise molecular responses in skeletal muscle21,22, we investigated potential interference to mTORC1 signalling following exercise protocols that participants were accustomed to via eight weeks of prior training. In contrast to previous studies in untrained or relatively training-unaccustomed participants14,16,17,18, we observed enhanced mTORC1 signalling after resistance training compared with concurrent exercise, including greater mTOR and p70S6K1 phosphorylation at 1 h post-exercise, and rps6 phosphorylation at 3 h post-exercise. These observations contrast with previous data, including our own20, showing no differences in mTORC1 signalling responses to single bouts of resistance exercise, performed alone or after a bout of continuous endurance exercise13. It has been suggested that any tendency for mTORC1 signalling responses (e.g., p70S6KThr389 phosphorylation) to be further enhanced by concurrent exercise (relative to resistance exercise alone) before training, as shown in a previous study14, were lessened when exercise was performed in a training-accustomed state13. Taken together, these data lend support to the notion the molecular signals initiated by exercise in skeletal muscle become more mode-specific with repeated training, and increases in post-exercise mTORC1 signalling with concurrent exercise may be attenuated when performed in a training-accustomed state.
While the observed mTORC1 signalling responses were consistent with the paradigm of enhanced mode-specificity of molecular responses with repeated training, the finding of greater AMPK phosphorylation following resistance exercise compared with concurrent exercise was unexpected, given the energy-sensing nature of AMPK signalling and its role in promoting an oxidative skeletal muscle phenotype48. This observation may suggest an adaptive response whereby endurance training rendered subjects in the concurrent training groups less susceptible to exercise-induced metabolic perturbation in skeletal muscle, manifesting in an attenuated post-exercise AMPK phosphorylation response. A similar phenomenon has been observed in human skeletal muscle after only 10 days of endurance training, whereby post-exercise increases in AMPK activity following a single pre-training exercise bout are attenuated compared with the same exercise bout performed before training49. It should also be acknowledged that while AMPK Thr172 phosphorylation alone does not necessarily reflect changes in AMPK activity per se, ACC Ser79 phosphorylation is generally accepted as a marker for AMPK activity50,51. Since we observed greater increases in ACC Ser79 phosphorylation with concurrent exercise versus resistance exercise alone during the post-training exercise trial, this may instead reflect further increases in AMPK activity in response to concurrent exercise. Nevertheless, the present data suggest further work is required to define the mode-specificity of AMPK signalling in skeletal muscle and the effect of repeated training on these responses.
In addition to mediating transient changes in translational efficiency, accumulating evidence suggests mTORC1 also plays a key role in regulating ribosome biogenesis (and therefore translational capacity) in skeletal muscle by regulating all three classes of RNA polymerases (RNA Pol-I to -III)25. In agreement with mTORC1 signalling responses, the phosphorylation of upstream regulators of RNA Pol-I-mediated rDNA transcription, including UBF and TIF-1A, was increased more by resistance exercise alone than when combined with endurance exercise in the form of either HIT or MICT. Previous work has demonstrated single sessions of resistance exercise to induce robust increases in TIF-1A Ser649 phosphorylation and UBF protein content in human skeletal muscle at 1 h post-exercise, both in untrained and trained states34. Moreover, whereas a single session of resistance exercise did not influence UBF Ser388 phosphorylation, this response was elevated in the basal state post-training34. The present data add to the growing body of evidence that resistance exercise is a potent stimulus for increasing the phosphorylation of regulators of Pol-I-mediated rDNA transcription, and suggest these early signalling responses may be similarly attenuated when resistance exercise is combined with endurance exercise. These responses also indicate an apparent disconnect between the upstream signalling responses in the post-training exercise trial related to 45S pre-rRNA transcription (i.e., TIF-1A and UBF phosphorylation), and the basal training-induced changes in markers of ribosomal content (i.e., total RNA and expression of mature rRNA species). While these responses appear paradoxical, they may suggest that although short-term concurrent training may optimise ribosome biogenesis adaptation versus resistance training performed alone, ribosome biogenesis may instead be further enhanced by longer-term resistance training performed alone. This notion aligns with recent discussion regarding the progression of adaptation with concurrent versus single-mode training, suggesting early adaptation to combined resistance and endurance training may initially be complimentary, whereas longer-term training exacerbates interference to hallmark resistance training adaptations52. Clearly, longer-term training studies are likely required to fully elucidate the effect of concurrent training versus resistance training alone on ribosome biogenesis adaptation in skeletal muscle.
Despite the present findings regarding signalling responses upstream of 45S pre-rRNA transcription, the expression of 45S pre-RNA, but not mature ribosome species, was increased only after concurrent exercise during the post-training exercise trial. Previous work in humans has reported basal increases in 45S pre-rRNA after 8 weeks of resistance training34, and 4 h after a single session of resistance exercise performed in both untrained and trained states33. Notably, post-exercise expression of 45S pre-rRNA was less pronounced in the trained compared with untrained state33. While no substantial basal changes in 45S pre-rRNA expression were observed in the present study, the change in 45S pre-rRNA levels between pre- and post-training was greater for both concurrent training groups compared with RT performed alone. Concurrent exercise also increased 45S pre-rRNA levels at 3 h post-exercise, with little effect of single-mode resistance exercise. These observations may be explained by the muscle sampling time points employed in the present study. Increased post-exercise 45S pre-rRNA levels have been previously shown 4 h after resistance exercise33, whereas a reduction in 45S rRNA levels has been demonstrated 1 h post- resistance exercise in trained, but not untrained, states34. The possibility therefore exists that resistance exercise may increase 45S rRNA expression at a later timepoint post-exercise, and the sampling time points employed herein were not extensive enough to measure any exercise-induced increases in 45S pre-rRNA expression.
The regulation of several Pol-I associated proteins was also measured at the transcriptional level, including TIF-1A, POLR1B, UBF, and cyclin D1. Concurrent exercise, irrespective of endurance training intensity, was sufficient to increase POLR1B mRNA expression at 3 h post-exercise, but only MICT+RT and RT alone increased TIF-IA mRNA content at this timepoint. Previous work in human skeletal muscle has demonstrated no effect of a single session of resistance exercise performed in either untrained or trained states on the mRNA expression of either TIF-1A or POLR1B at either 1 h34 or 4 h33 post-exercise. Eight weeks of resistance training has previously been shown to increase basal UBF mRNA expression, which was reduced 1 h following a single session of resistance exercise performed post-training34. Although we observed no basal training-induced increases in UBF mRNA expression for any training group, a similar reduction in UBF mRNA content was noted 3 h post-exercise for the RT group. Increased cyclin D1 mRNA was also seen at rest post-training for the HIT+RT group, which was maintained at 3 h post-exercise. Figueiredo et al.34 have shown eight weeks of resistance training decreased post-training levels of cyclin D1 mRNA compared with pre-training, with a small increase induced at 1 h post-exercise by a single session of post-training resistance exercise. It therefore appears HIT is a more potent stimulus for increasing levels of cyclin D1 mRNA compared with resistance exercise alone or MICT, although an acute reduction in cyclin D1 protein levels was also seen 1 h following a single bout of HIT+RT. Previous work has shown increases in cyclin D1 mRNA during long-term (3 months) resistance training53, which may suggest an increase in satellite cell activation and proliferation during the training intervention53,54, although direct measures of these markers were not made in the present study.
The rRNA primers used in the present study were specifically designed to differentiate between mature rRNA expression and the expression of these sequences when still bound to the polycistrionic 45S rRNA precursor (i.e., 5.8S, 18S and 28S [span] rRNA)34. Previous work using these primer sequences has shown basal training-induced increases in mature rRNA expression did not occur concomitantly with increased expression of rRNA transcripts still bound to the 45S precursor (i.e., 5.8S, 18S and 28S [span]), suggesting a training-induced increase in mature rRNA content, rather than increased 45S precursor expression34. In contrast, we observed simultaneous post-exercise increases in the expression of both mature rRNA transcripts and those still bound to the 45S precursor (i.e., ‘span’ rRNA transcripts). It is therefore possible our observed changes in these markers may be reflective solely of changes in 45S pre-rRNA content, and not the mature forms of these rRNAs. However, it is also possible this may relate to the post-exercise time course examined in the present study. In support of this notion, it was shown that a single session of resistance exercise was sufficient to increase only the expression of rRNA transcripts still bound to the 45S pre-rRNA, and not mature rRNA species, even after 48 h of post-exercise recovery55. It is therefore plausible that the post-exercise time courses examined in the present study were not extensive enough to measure early post-exercise changes in mature rRNA expression. Clearly, further work is required to investigate the time course of rRNA regulation with training in human skeletal muscle.
Although we have investigated various upstream regulators of 45S pre-rRNA transcription, it is possible other factors may have been differentially regulated by concurrent versus single-mode resistance training and may have contributed to the observed changes in ribosome biogenesis markers. For example, CAD (carbamoyl-phosphate synthetase 2, aspartate transcarbamoylase, dihydroorotase) is directly phosphorylated by p70S6K1 and controls the first three steps in de novo pyrimidine synthesis56, a necessary process for accommodating the increased demand for RNA and DNA synthesis to support cellular growth. To our knowledge, the regulation of CAD has, however, not been investigated in the context of training-induced skeletal muscle hypertrophy in humans. Future studies should also consider the potential role of CAD in the regulation of skeletal muscle growth in response to resistance and/or concurrent training.
Conclusions
This is the first study to simultaneously investigate markers of ribosome biogenesis and mTORC1 signalling in human skeletal muscle following concurrent training compared with single-mode resistance training. Contrary to our hypotheses, and recent work in humans33,34, we noted little evidence of ribosome biogenesis in skeletal muscle following eight weeks of resistance training. Rather, training-induced increases in markers of ribosome biogenesis, tended to be greater following concurrent training and were independent of the endurance training intensity employed. This occurred despite a single session of resistance exercise, when performed post-training, being a more potent stimulus for both mTORC1 signalling and phosphorylation of upstream regulators of RNA Pol-1-mediated rDNA transcription (i.e., TIF-1A and UBF). An apparent disconnect was noted between training-induced changes in muscle fibre CSA, of which the small increase in type I fibre CSA induced by resistance training was attenuated when combined with HIT, and changes in total skeletal muscle RNA content. Overall, the present data suggest single-mode resistance exercise performed in a training-accustomed state preferentially induces mTORC1 and ribosome biogenesis-related signalling in skeletal muscle compared with concurrent exercise; however, this is not associated with basal post-training increases in markers of ribosome biogenesis. The observation that both mTORC1 and ribosome biogenesis-related signalling were impaired in response to the final training session of the study for both forms of concurrent exercise, relative to resistance exercise performed alone, suggests resistance training may be a more potent stimulus for ribosome biogenesis and muscle hypertrophy if training were continued longer-term. Further work in human exercise models that stimulate more robust skeletal muscle hypertrophy (e.g., high-volume resistance training performed to failure), together with longer training periods, are likely needed to definitively elucidate the role of ribosome biogenesis in adaptation to resistance training, and subsequently any potential interference to these responses with concurrent training.
Methods
Ethical approval
All study procedures were approved by the Victoria University Human Research Ethics Committee (HRE 13-309). After being fully informed of study procedures and screening for possible exclusion criteria, participants provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations of the Victoria University Human Research Ethics Committee.
Experimental overview
Participant details and procedures performed in this study have been previously described44; however, these are briefly summarised as follows. The study employed a repeated-measures, parallel-group design (Fig. 8A). After preliminary testing for maximal (1-RM) strength, aerobic fitness (\(\dot{\text{V}}\text{O}\) 2peak, the lactate threshold [LT] and peak aerobic power [Wpeak]), and body composition (dual-energy x-ray absorptiometry [DXA]), participants were ranked by baseline 1-RM leg press strength and randomly allocated to one of three training groups. Each group performed training sessions that consisted of either 1) high-intensity interval training (HIT) cycling combined with resistance training (HIT+RT group, n = 8), 2) moderate-intensity continuous training (MICT) cycling combined with resistance training (MICT+RT group, n = 7) or 3) resistance training performed alone (RT group, n = 8).
Study overview (A) and timelines for the final training session (B). Participants first completed 8 weeks of either resistance training (RT) alone, or RT combined with either high-intensity interval training (HIT+RT) or moderate-intensity continuous training (MICT+RT). For the final training session (B), participants completed the RE protocol alone (i) or after a 15-min recovery following the completion of either HIT (ii) or work-matched MICT (iii) cycling. Muscle biopsies were obtained from the vastus lateralis at rest before training, and immediately before beginning the final training session, and 1 h and 3 h after completion of RE.
After preliminary testing, and immediately prior to the first training session (i.e., at least 72 h after completion of preliminary testing), a resting muscle biopsy (PRE-T) was obtained from the vastus lateralis using the percutaneous needle biopsy technique57 modified with suction58. Participants then completed 8 weeks of group-specific training performed three times per week. Between 48 and 72 h after completing the post-training 1-RM strength testing, participants underwent a final group-specific training session (Fig. 8B) whereby early post-exercise molecular responses in skeletal muscle were measured in a training-accustomed state. Three additional biopsies [at rest (POST-T), and 1 h (+1 h) and 3 h (+3 h) post-exercise] were obtained during the final group-specific training session.
Training intervention
The training intervention in this study has previously been described in detail44. Briefly, participants began the 8-week training intervention 3 to 5 days after completion of preliminary testing. All training groups performed an identical resistance training program on non-consecutive days (typically Monday, Wednesday, and Friday), with the HIT+RT and MICT+RT groups completing the corresponding form of endurance exercise 10 min prior to commencing each resistance training session.
Final training session
Two or three days after completion of the training intervention and post-testing, participants perfomed a final group-specific training session (Fig. 8B) whereby early post-exercise skeletal muscle responses were measured in a training-accustomed state. Participants reported to the laboratory after an overnight (~8–10 h) fast. After resting quietly for ~15 min upon arrival at the laboratory, a venous cathether was inserted into an anticubital forearm vein and a resting blood sample was obtained. A resting, post-training (POST-T) muscle biopsy was then taken from the vastus lateralis muscle (described subsequently). Participants in the RT group waited quietly for 10 min after the POST-T biopsy and then completed a standardised resistance exercise protocol (8 × 5 leg press repetitions at 80% of the post-training 1-RM, three minutes of recovery between sets). Participants in the HIT+RT and MICT+RT groups preceded the standardised RT with either HIT (10 × 2-min intervals at 140% of the post-training LT, 1 min passive recovery between intervals) or work- and duration-matched MICT cycling (30 min at 93.3% post-training LT), respectively. Fifteen minutes of passive recovery was allowed between completion of either HIT or MICT and the subsequent resistance exercise bout. Each cycling bout was performed after a standardised warm-up ride at 75 W for 5 min. After completion of resistance exercise, participants rested quietly in the laboratory and additional biopsies were obtained after 1 (+1 h) and 3 h (+3 h) of recovery. Venous blood samples were also obtained at regular intervals during cycling and following recovery from both cycling and resistance exercise (Fig. 8B).
Muscle sampling
After administration of local anaesthesia (1% Xylocaine), a small incision (~7 mm in length) was made through the skin, subcutaneous tissue, and fascia overlying the vastus lateralis muscle for each subsequent biopsy. A 5-mm Bergström needle was then inserted into the muscle and a small portion of muscle tissue (~50–400 mg) removed. All biopsies were obtained from separate incision sites in a distal-to-proximal fashion on the same leg as the pre-training biopsy. Muscle samples were blotted on filter paper to remove excess blood, immediately frozen in liquid nitrogen, and stored at −80 °C until subsequent analysis. A small portion of each biopsy sample (~20 mg) was embedded in Tissue-Tek (Sakura, Finetek, NL), frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C for subsequent immunofluorescence analysis.
Western blotting
Approximately 5 mg of frozen muscle tissue was homogenised in lysis buffer (0.125 M Tris-HCl, 4% SDS, 10% Glycerol, 10 mM EGTA, 0.1 M DTT, 1% protease/phosphatase inhibitor cocktail), left for 1 h at room temperature, and then stored overnight at −80 °C. The following morning, samples were thawed and the protein concentration determined (Red 660 Protein Assay Kit, G-Biosciences, St. Louis, MO). Bromophenol blue (0.1%) was then added to each sample, which were then stored at −80 °C until subsequent analysis. Proteins (8 µg) were separated by SDS-PAGE using 6–12% acrylamide pre-cast gels (TGX Stain Free, Bio-Rad laboratories, Hercules, CA) in 1 × running buffer (25 mM Tris, 192 mM Glycine, 0.1% SDS), and transferred to polyvinylidine fluoride (PVDF) membranes (Bio-Rad laboratories, Hercules, CA) using a semi-dry transfer system (Trans Blot Turbo, Bio-Rad laboratories, Hercules, CA) for 7 min at 25 V. After transfer, membranes were blocked with 5% skim milk in 1×TBST (200 mM Tris, 1.5 M NaCl, 0.05% Tween 20) for 1 h at room temperature, washed with 1×TBST (5 × 5 min), and incubated with primary antibody solution (5% BSA [bovine serum albumin], 0.05% Na Azide in 1×TBST) overnight at 4 °C. Primary antibodies for phosphorylated (p-) p-mTORSer2448 (1:1000; #5536), mTOR (1:1000), p-p70S6K1Thr389 (1:1000; #9234), p70S6K1 (1:1000), p-4E-BP1Thr37/46 (1:1000; #2855), 4E-BP1 (1:1000; #9452), p-AMPKThr172 (1:1000; #2535), AMPK (1:1000; #2532), p-rps6Ser235/236 (1:750; #4856), rps6 (1:1000; #2217) and p-ACCSer79 (1:1000; #3661) were from Cell Signalling Technology (Danvers, MA), p-UBFSer388 (1:1000; sc-21637-R), UBF (1:000; sc-9131) and cyclin D1 (1:1000; sc-450) were from Santa Cruz Biotechnology (Dallas, TX), and p-RRN3 (TIF-1A)Ser649 (1:1000; ab138651) and TIF-1A (1:1000; ab70560) were from Abcam (Cambridge, UK). The following morning, membranes were washed again with 1×TBST and incubated with a secondary antibody (Perkin Elmer, Waltham, MA, #NEF812001EA; 1:50000 or 1:100000 in 5% skim milk and 1×TBST) for 1 h at room temperature. After washing again with 1×TBST, proteins were detected with chemiluminescence (SuperSignalTM West Femto Maximum Sensitivity Substrate, Thermo Fisher Scientific, Waltham, MA) and quantified via densitometry (Image Lab 5.0, Bio-Rad laboratories, Hercules, CA). Representative western blot images for each protein target analysed are shown in Fig. 9. All sample timepoints for each participant were run on the same gel and normalised to both an internal pooled sample present on each gel, and the total protein content of each lane using a stain-free imaging system (Chemi DocTM MP, Bio-Rad laboratories, Hercules, CA). Phosphorylated proteins were then expressed relative to the total amount of each respective protein (with the exception of phosphorylated ACCSer79, which was normalised only to the total protein content of each lane due to technical difficulties when measuring total ACC protein).
Representative western blots for the phosphorylation (p-) and total protein content of signalling proteins before (PRE-T) and after (POST-T) eight weeks of either RT alone, or RT combined with either high-intensity interval training (HIT+RT) or moderate-intensity continuous training (MICT+RT), and 1 h (+1 h) and 3 h (+3 h) after a single exercise bout performed post-training. Cropped western blot images are displayed for clarity of presentation, and full-length western blot images are presented in supplementary information.
Real-time quantitative PCR (qPCR)
RNA extraction
Total RNA (1145 ± 740 ng; mean ± SD) was extracted from approximately 25 mg of muscle tissue using TRI Reagent® (Sigma Aldrich, St. Louis, MO) according to the manufacturer’s protocol. Muscle samples were firstly homogenised in 500 μL of TRI Reagent® using a Tissue Lyser II and 5 mm stainless steel beads (Qiagen, Venlo, Limburg, Netherlands) for 120 s at 30 Hz. After resting for 5 min on ice, 50 μL of 1-bromo-3-chloropropane (BCP) was added to the tube, inverted for 30 s to mix, and then rested for 10 min at room temperature. The homogenate was then centrifuged for 15 min at 13,000 rpm and the upper transparent phase transferred to another tube. Isopropanol (400 μL) was added to the tube, inverted briefly to mix, and stored overnight at −20 °C to precipitate the RNA. After overnight incubation, the solution was centrifuged for 60 min at 13,000 rpm and at 4 °C to pellet the RNA. The RNA pellet was washed twice by centrifugation in 75% ethanol/nuclease-free water (NFW) for 15 min at 13,000 rpm, allowed to air-dry, and then dissolved in 15 μL of NFW (Ambion Inc., Austin, TX). The quantity and quality of RNA was subsequently determined using a spectrophotometer (NanoDrop One, Thermo Scientific, Wilmington, DE). The purity of RNA was assessed using the ratio between the absorbance at 260 nm and absorbance at 280 nm (mean ± SD; 2.37 ± 0.43), and the ratio between the absorbance at 260 nm and absorbance at 230 nm (1.71 ± 0.42). The total skeletal muscle RNA concentration was calculated based on the total RNA yield relative to the wet weight of the muscle sample.
Reverse transcription
For mRNA analysis, first-strand cDNA was generated from 1 µg RNA in 20 μL reaction buffer using the iScript® cDNA synthesis kit (Bio-Rad laboratories, Hercules, CA) according to manufacturer’s protocol, with each reaction comprising 4 μL 5 × iScript reaction mix, 1 μL iScript Reverse Transcriptase, 5 μL NFW and 10 μL of RNA sample (100 ng/μL). Reverse transcription was then performed with the following conditions: 5 min at 25 °C to anneal primers, 30 min at 42 °C for the extension phase, and 5 min at 85 °C. Following reverse transcription, samples were DNase-treated (Life Technologies, Carlsbad, CA) and cDNA was stored at −20 °C until further analysis.
Real-time quantitative PCR (qPCR)
Real-time PCR was performed using a Realplex2 PCR system (Eppendorf, Hamburg, Germany) to measure mRNA levels of UBF, TIF-1A, cyclin D1, POLR1B, and commonly used reference genes GAPDH (glyceraldehyde 3-phosphate dehydrogenase), cyclophilin (also known as peptidyl-prolylcis-trans isomerase), β2M (beta-2 microglobulin) and TBP (TATA binding protein). Target rRNAs were the mature ribosome species 5.8S, 18S and 28S. Since primers specific for these mature rRNA sequences will also amplify pre-RNA transcripts (i.e., the 45S pre-rRNA), we used specifically designed primers (QIAGEN, Venlo, Limburg, The Netherlands) to distinguish between mature rRNA species and those still bound to the 45S pre-rRNA transcript, as previously described34. Briefly, primers were designed specifically for pre-rRNA sequences spanning the 5′end external/internal transcribed spacer regions (ETS and ITS, respectively) of the 45S pre-RNA transcript and the internal regions of mature rRNA sequences (i.e., 18S-ETS, 5.8S-ITS, and 28S-ETS). For clarity, primers amplifying the mature rRNA transcripts are henceforth designated as ‘mature’ transcripts (e.g., 18S rRNA [mature]), as opposed to those primers amplifying rRNA sequences bound to the 45S rRNA precursor, henceforth designated as ‘span’ transcripts (e.g., 18S rRNA [span]). A specific primer for the initial region of the 5′ end of the 45S pre-rRNA transcript was used to measure 45S pre-rRNA expression levels34. Standard and melting curves were performed for all primers to ensure both single-product and amplification efficiency. Details for all primers used are provided in Table 6 (mRNA) and Table 7 (rRNA).
Each PCR reaction was performed in duplicate using a robotic pipetting machine (EpMotion 2100, Eppendorf, Hamburg, Germany) in a final reaction volume of 10 μL containing 5.0 μL 2× SYBR green (Bio-Rad Laboratories, Hercules, CA), 0.6 μL PCR primers (diluted to 15 µM; Sigma Aldrich, St. Louis, MO), 0.4 μL NFW and 4 μL cDNA sample (diluted to 5 ng/μL). Conditions for the PCR reactions were: 3 min at 95 °C, 40 cycles of 15 sec at 95 °C/1 min at 60 °C, one cycle of 15 sec at 95 °C/15 sec at 60 °C, and a ramp for 20 min to 95 °C. Each plate was briefly centrifuged before loading into the PCR machine. To compensate for variations in input RNA amounts and efficiency of the reverse transcription, mRNA data were quantified using the 2−∆∆CT method59 and normalised to the geometric mean60 of the three most stable housekeeping genes analysed (cyclophillin, β2M and TBP), determined as previously described61.
Immunohistochemistry
Muscle cross-sections (10 µM) were cut at −20 °C using a cryostat (Microm HM 550, Thermo Fisher Scientific, Waltham, MA), mounted on uncoated glass slides, and air-dried for 20 min at room temperature. Sections were then rinsed briefly with 1 × PBS (0.1 M; Sigma Aldrich, St Louis, MO), fixed with cold paraformaldehyde (4% in 1 × PBS) for 10 min at room temperature, rinsed three times with 1 × PBS, incubated in 0.5% TritonX in 1 × PBS for 5 min at room temperature, rinsed again three times with 1 × PBS, and then blocked for 1 h at room temperature in a 3% BSA solution in 1 × PBS. After blocking, sections were then incubated with a primary antibody for myosin heavy chain type I (A4.840, Developmental Studies Hybridoma Bank, University of Iowa, IA), diluted 1:25 in 3% BSA/PBS overnight at 4 °C. The following morning, sections were washed four times in 1 × PBS for 10 min each, before incubating with a secondary antibody (Alexa Fluor® 488 conjugate Goat anti-mouse IgM, cat. no. A-21042, Thermo Fisher Scientific, Waltham, MA) diluted 1:200 in 3% BSA/PBS for 2 h at room temperature. Sections were again washed four times in 1 × PBS for 10 min each, before incubation with Wheat Germ Agglutinin (WGA) (Alexa Fluor® 594 Conjugate; cat. no. W11262, Thermo Fisher Scientific, Waltham, MA), diluted to 1:100 in 1 × PBS (from a 1.25 mg/mL stock solution), for 15 min at room temperature. Sections were washed again 4 times with 1 × PBS for 3 min each, blotted dry with a Kim-Wipe, and FlouroshieldTM (cat. no. F6182; Sigma Aldrich, St Louis, MO) added to each section before the coverslip was mounted. Stained muscle sections were air-dried for ~2 h and viewed with an Olympus BX51 microscope coupled with an Olympus DP72 camera for flourescence detection (Olympus, Shinjuku, Japan). Images were captured with a 10× objective and analysed using Image Pro Premier software (version 9.1; Media Cybernetics, Rockville, MD). Analysis was completed by an investigator blinded to all groups and time points. For each subject, muscle fibre CSA was determined for both type I and type II muscle fibres. For the RT, HIT+RT and MICT+RT groups, a total of 107 ± 61, 112 ± 67, and 84 ± 73 (mean ± SD) type I fibres and 154 ± 72, 136 ± 80, and 144 ± 76 (mean ± SD) type II fibres were included for analysis, respectively.
Statistical analyses
The effect of training group on outcomes was analysed using a combination of both traditional and magnitude-based statistical analyses. Western blot, qPCR and immunohistochemistry data were log-transformed before analysis to reduce non-uniformity of error62. Data were firstly analysed via a two-way (time × group) analysis of variance with repeated-measures (RM-ANOVA) (SPSS, Version 21, IBM Corporation, New York, NY). To quantify the magnitude of within- and between-group differences for dependent variables, a magnitude-based approach to inferences using the standardised difference (effect size, ES) was used62. The magnitude of effects were defined according to thresholds suggested by Hopkins62, whereby <0.2 = trivial, 0.2–0.6 = small, 0.6–1.2 = moderate, 1.2–2.0 = large, 2.0–4.0 = very large and >4.0 = extremely large effects. Lacking information on the smallest meaningful effect for changes in protein phosphorylation and gene expression, the threshold for the smallest worthwhile effect was defined as an ES of 0.4, rather than the conventional threshold of 0.220. Magnitude-based inferences about effects were made by qualifying the effects with probabilities reflecting the uncertainty in the magnitude of the true effect63. Effects that were deemed substantial in magnitude (and therefore meaningful) were those at least 75% ‘likely’ to exceed the smallest worthwhile effect (according to the overlap between the uncertainty in the magnitude of the true effect and the smallest worthwhile change63). Exact P values were also determined for each comparison, derived from paired (for within-group comparisons) or unpaired (for between-group comparisons) t-tests, with a Bonferroni correction applied to correct for multiple comparisons (SPSS, Version 21, IBM Corporation, New York, NY). A summary of all within- and between-group comparisons for this study are presented in Supplementary Tables 1 and 2, respectively. Physiological (blood lactate, blood glucose, heart rate) and psychological (rating of perceived exertion [RPE]) responses to exercise are reported as mean values ± SD, whereas protein phosphorylation and gene expression data are reported as mean within- and between-condition percentage differences ± 90% CL.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Leveritt, M., Abernethy, P. J., Barry, B. K. & Logan, P. A. Concurrent strength and endurance training. A review. Sports Med 28, 413–427 (1999).
Kraemer, W. J. et al. Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol (1985) 78, 976–989 (1995).
Hickson, R. C. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol 45, 255–263 (1980).
Bell, G. J., Syrotuik, D., Martin, T. P., Burnham, R. & Quinney, H. A. Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur J Appl Physiol 81, 418–427 (2000).
Bodine, S. C. et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3, 1014–1019, https://doi.org/10.1038/ncb1101-1014 (2001).
Drummond, M. J. et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587, 1535–1546, https://doi.org/10.1113/jphysiol.2008.163816 (2009).
Kimball, S. R. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc 38, 1958–1964, https://doi.org/10.1249/01.mss.0000233796.16411.13 (2006).
Atherton, P. J. et al. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 19, 786–788, https://doi.org/10.1096/fj.04-2179fje (2005).
Apro, W. et al. Resistance exercise-induced S6K1 kinase activity is not inhibited in human skeletal muscle despite prior activation of AMPK by high-intensity interval cycling. Am J Physiol Endocrinol Metab 308, E470–481, https://doi.org/10.1152/ajpendo.00486.2014 (2015).
Apro, W., Wang, L., Ponten, M., Blomstrand, E. & Sahlin, K. Resistance exercise induced mTORC1 signalling is not impaired by subsequent endurance exercise in human skeletal muscle. Am J Physiol Endocrinol Metab 305, E22–32, https://doi.org/10.1152/ajpendo.00091.2013 (2013).
Coffey, V. G. et al. Effect of consecutive repeated sprint and resistance exercise bouts on acute adaptive responses in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297, R1441–1451, 00351.2009 (2009).
Coffey, V. G., Pilegaard, H., Garnham, A. P., O’Brien, B. J. & Hawley, J. A. Consecutive bouts of diverse contractile activity alter acute responses in human skeletal muscle. J Appl Physiol 106, 1187–1197, 91221.2008 (2009).
Fernandez-Gonzalo, R., Lundberg, T. R. & Tesch, P. A. Acute molecular responses in untrained and trained muscle subjected to aerobic and resistance exercise training versus resistance training alone. Acta Physiol (Oxf) 209, 283–294, https://doi.org/10.1111/apha.12174 (2013).
Lundberg, T. R., Fernandez-Gonzalo, R., Gustafsson, T. & Tesch, P. A. Aerobic exercise alters skeletal muscle molecular responses to resistance exercise. Med Sci Sports Exerc 44, 1680–1688, https://doi.org/10.1249/MSS.0b013e318256fbe8 (2012).
Lundberg, T. R., Fernandez-Gonzalo, R. & Tesch, P. A. Exercise-induced AMPK activation does not interfere with muscle hypertrophy in response to resistance training in men. J Appl Physiol (1985) 116, 611–620, https://doi.org/10.1152/japplphysiol.01082.2013 (2014).
Donges, C. E. et al. Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men. J Appl Physiol 112, 1992–2001, japplphysiol.00166.2012 (2012).
Pugh, J. K., Faulkner, S. H., Jackson, A. P., King, J. A. & Nimmo, M. A. Acute molecular responses to concurrent resistance and high-intensity interval exercise in untrained skeletal muscle. Physiological reports 3, https://doi.org/10.14814/phy2.12364 (2015).
Carrithers, J. A., Carroll, C. C., Coker, R. H., Sullivan, D. H. & Trappe, T. A. Concurrent exercise and muscle protein synthesis: implications for exercise countermeasures in space. Aviat Space Environ Med 78, 457–462 (2007).
Wilson, J. M. et al. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res 26, 2293–2307, https://doi.org/10.1519/JSC.0b013e31823a3e2d (2012).
Fyfe, J. J., Bishop, D. J., Zacharewicz, E., Russell, A. P. & Stepto, N. K. Concurrent exercise incorporating high-intensity interval or continuous training modulates mTORC1 signalling and microRNA expression in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 310, R1297–1311, https://doi.org/10.1152/ajpregu.00479.2015 (2016).
Vissing, K. et al. Differentiated mTOR but not AMPK signaling after strength vs endurance exercise in training-accustomed individuals. Scand J Med Sci Sports 23, 355–366, https://doi.org/10.1111/j.1600-0838.2011.01395.x (2011).
Wilkinson, S. B. et al. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586, 3701–3717, jphysiol.2008.153916 (2008).
Fyfe, J. J., Bishop, D. J. & Stepto, N. K. Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med 44, 743–762, https://doi.org/10.1007/s40279-014-0162-1 (2014).
Chaillou, T., Kirby, T. J. & McCarthy, J. J. Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. Journal of cellular physiology 229, 1584–1594, https://doi.org/10.1002/jcp.24604 (2014).
Iadevaia, V., Liu, R. & Proud, C. G. mTORC1 signaling controls multiple steps in ribosome biogenesis. Seminars in cell & developmental biology 36, 113–120, https://doi.org/10.1016/j.semcdb.2014.08.004 (2014).
Thomson, E., Ferreira-Cerca, S. & Hurt, E. Eukaryotic ribosome biogenesis at a glance. J Cell Sci 126, 4815–4821, https://doi.org/10.1242/jcs.111948 (2013).
Moss, T. & Stefanovsky, V. Y. Promotion and regulation of ribosomal transcription in eukaryotes by RNA polymerase I. Progress in nucleic acid research and molecular biology 50, 25–66 (1995).
Mayer, C., Zhao, J., Yuan, X. & Grummt, I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev 18, 423–434, https://doi.org/10.1101/gad.285504 (2004).
Hannan, K. M. et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol 23, 8862–8877 (2003).
Voit, R. & Grummt, I. Phosphorylation of UBF at serine 388 is required for interaction with RNA polymerase I and activation of rDNA transcription. Proc Natl Acad Sci USA 98, 13631–13636, https://doi.org/10.1073/pnas.231071698 (2001).
Voit, R., Hoffmann, M. & Grummt, I. Phosphorylation by G1-specific cdk-cyclin complexes activates the nucleolar transcription factor UBF. EMBO J 18, 1891–1899, https://doi.org/10.1093/emboj/18.7.1891 (1999).
Kantidakis, T., Ramsbottom, B. A., Birch, J. L., Dowding, S. N. & White, R. J. mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci USA 107, 11823–11828, https://doi.org/10.1073/pnas.1005188107 (2010).
Nader, G. A. et al. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (1985) 116, 693–702, https://doi.org/10.1152/japplphysiol.01366.2013 (2014).
Figueiredo, V. C. et al. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309, E72–83, https://doi.org/10.1152/ajpendo.00050.2015 (2015).
Stec, M. J., Mayhew, D. L. & Bamman, M. M. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol (1985) 119, 851–857, https://doi.org/10.1152/japplphysiol.00489.2015 (2015).
von Walden, F., Casagrande, V., Ostlund Farrants, A. K. & Nader, G. A. Mechanical loading induces the expression of a Pol I regulon at the onset of skeletal muscle hypertrophy. Am J Physiol Cell Physiol 302, C1523–1530, https://doi.org/10.1152/ajpcell.00460.2011 (2012).
Chaillou, T. et al. Hypoxia transiently affects skeletal muscle hypertrophy in a functional overload model. Am J Physiol Regul Integr Comp Physiol 302, R643–654, https://doi.org/10.1152/ajpregu.00262.2011 (2012).
Chaillou, T., Lee, J. D., England, J. H., Esser, K. A. & McCarthy, J. J. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol (1985) 115, 1065–1074, https://doi.org/10.1152/japplphysiol.00611.2013 (2013).
Miyazaki, M., McCarthy, J. J., Fedele, M. J. & Esser, K. A. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J Physiol 589, 1831–1846, https://doi.org/10.1113/jphysiol.2011.205658 (2011).
Goodman, C. A. et al. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589, 5485–5501, https://doi.org/10.1113/jphysiol.2011.218255 (2011).
Adams, G. R., Caiozzo, V. J., Haddad, F. & Baldwin, K. M. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 283, C1182–1195, https://doi.org/10.1152/ajpcell.00173.2002 (2002).
Kirby, T. J. et al. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol (1985) Aug 15 119, 321–327, https://doi.org/10.1152/japplphysiol.00296.2015 (2015).
Brook, M. S. et al. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol 594, 7399–7417, https://doi.org/10.1113/JP272857 (2016).
Fyfe, J. J., Bartlett, J. D., Hanson, E. D., Stepto, N. K. & Bishop, D. J. Endurance training intensity does not mediate interference to maximal lower-body strength gain during short-term concurrent training. Frontiers in physiology Nov 3,7, https://doi.org/10.3389/fphys.2016.00487 (2016).
Haddad, F., Baldwin, K. M. & Tesch, P. A. Pretranslational markers of contractile protein expression in human skeletal muscle: effect of limb unloading plus resistance exercise. J Appl Physiol (1985) 98, 46–52, https://doi.org/10.1152/japplphysiol.00553.2004 (2005).
Nakada, S., Ogasawara, R., Kawada, S., Maekawa, T. & Ishii, N. Correlation between Ribosome Biogenesis and the Magnitude of Hypertrophy in Overloaded Skeletal Muscle. PLoS One 11, https://doi.org/10.1371/journal.pone.0147284. (2016).
Goodman, C. A. et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25, 1028–1039, fj.10-168799 (2011).
McGee, S. L. & Hargreaves, M. AMPK-mediated regulation of transcription in skeletal muscle. Clin Sci (Lond) 118, 507–518, CS20090533 (2010).
McConell, G. K. et al. Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol 568, 665–676, jphysiol.2005.089839 (2005).
Ha, J., Daniel, S., Broyles, S. S. & Kim, K. H. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem 269, 22162–22168 (1994).
Park, S. H. et al. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol (1985) 92, 2475–2482, https://doi.org/10.1152/japplphysiol.00071.2002 (2002).
Coffey, V. G. & Hawley, J. A. Concurrent exercise training: do opposites distract? J Physiol 595, 2883–2896, https://doi.org/10.1113/JP272270 (2016).
Kadi, F. et al. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558, 1005–1012, https://doi.org/10.1113/jphysiol.2004.065904 (2004).
Adams, G. R., Haddad, F. & Baldwin, K. M. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol (1985) 87, 1705–1712 (1999).
Figueiredo, V. C. et al. Impact of resistance exercise on ribosome biogenesis is acutely regulated by post-exercise recovery strategies. Physiological reports 4, https://doi.org/10.14814/phy2.12670 (2016).
Ben-Sahra, I., Howell, J. J., Asara, J. M. & Manning, B. D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328, https://doi.org/10.1126/science.1228792 (2013).
Bergstrom, J. Muscle electrolytes in man. Scand J Clin Lab Invest 68, 1–110 (1962).
Evans, W. J., Phinney, S. D. & Young, V. R. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14, 101–102 (1982).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25, 402–408, https://doi.org/10.1006/meth.2001.1262 (2001).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3, RESEARCH0034 (2002).
Mane, V. P., Heuer, M. A., Hillyer, P., Navarro, M. B. & Rabin, R. L. Systematic method for determining an ideal housekeeping gene for real-time PCR analysis. J Biomol Tech 19, 342–347 (2008).
Hopkins, W. G., Marshall, S. W., Batterham, A. M. & Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 41, 3–13, https://doi.org/10.1249/MSS.0b013e31818cb278 (2009).
Batterham, A. M. & Hopkins, W. G. Making Meaningful Inferences About Magnitudes. Sportscience, sportsci.org/jour/05/ambwgh.htm (2005).
Thomson, D. M., Fick, C. A. & Gordon, S. E. AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J Appl Physiol 104, 625–632, 00915.2007 (2008).
Acknowledgements
We gratefully acknowledge the efforts of the participants, without whom this study would not have been possible. We also acknowledge Dr Chris Shaw (Deakin University) for technical assistance with the immunofluorescence analysis. This study was supported, in part, by a grant from the Gatorade Sports Science Institute (GSSI) awarded to J.J.F.
Author information
Authors and Affiliations
Contributions
Study design was performed by J.J.F., J.D.B., E.D.H., D.J.B. and N.K.S. Data collection was performed by J.J.F, M.J.A and A.P.G. Analysis and interpretation of data was performed by J.J.F., J.D.B., E.D.H., D.J.B. and N.K.S. The manuscript was written by J.J.F., D.J.B., and N.K.S., while J.D.B., E.D.H., M.J.A and A.P.G. critically revised the manuscript. All authors approved the final version of the manuscript. All data collection and data analysis for this study was conducted and performed in the exercise physiology and biochemistry laboratories at Victoria University, Footscray Park campus, Melbourne Australia.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fyfe, J.J., Bishop, D.J., Bartlett, J.D. et al. Enhanced skeletal muscle ribosome biogenesis, yet attenuated mTORC1 and ribosome biogenesis-related signalling, following short-term concurrent versus single-mode resistance training. Sci Rep 8, 560 (2018). https://doi.org/10.1038/s41598-017-18887-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18887-6
This article is cited by
-
The Plateau in Muscle Growth with Resistance Training: An Exploration of Possible Mechanisms
Sports Medicine (2024)
-
The Effects of Concurrent Aerobic and Strength Training on Muscle Fiber Hypertrophy: A Systematic Review and Meta-Analysis
Sports Medicine (2022)
-
Development of Maximal Dynamic Strength During Concurrent Resistance and Endurance Training in Untrained, Moderately Trained, and Trained Individuals: A Systematic Review and Meta-analysis
Sports Medicine (2021)
-
The skeletal muscle fiber: a mechanically sensitive cell
European Journal of Applied Physiology (2019)
-
Adaptations to Concurrent Training in Combination with High Protein Availability: A Comparative Trial in Healthy, Recreationally Active Men
Sports Medicine (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.