Key Points
-
Seminal plasma contains a large number of tissue-specific proteins secreted by individual organs of the male reproductive system
-
Male reproductive system disorders result in altered composition of the seminal plasma proteome
-
High concentrations of organ-specific proteins enable their accurate quantification by mass spectrometry, facilitating biomarker discovery
-
As well as being a promising biological fluid for biomarker discovery, seminal plasma might find a unique niche as a clinical diagnostic fluid
-
Because cancer-specific proteins can appear earlier in seminal plasma than in blood serum, seminal plasma could facilitate the early diagnosis of prostate and testicular cancers
Abstract
Molecular biomarkers hold promise to advance the noninvasive diagnosis of male reproductive system disorders and facilitate the identification and management of these conditions through screening, early diagnosis and more accurate prognosis. Seminal plasma has great potential as a proximal fluid for protein biomarker discovery and as a clinical sample for noninvasive diagnostics. The seminal plasma proteome contains thousands of proteins and includes a large number of tissue-specific proteins that might accurately indicate a pathological process in the tissue of origin. Potential protein biomarkers for male reproductive system disorders are more abundant in seminal plasma than in blood serum or urine, and, therefore, are more easily identified and quantified in semen by mass spectrometry and other techniques. These methods have enabled elaboration of the composition of the seminal plasma proteome and the tissue specificity of seminal plasma proteins. Strategies have been developed to discover protein biomarkers in seminal plasma through integrated 'omics' approaches. Biomarkers of male infertility and prostate cancer are now emerging, and it is evident that seminal plasma has the potential to complement other diagnostic tools available in urology clinics.
Similar content being viewed by others
Introduction
Urogenital diseases affect the quality of life of many men. Prostatitis-like symptoms are diagnosed in as many as one in six men,1 and approximately the same fraction will develop prostate cancer at some point in their lifetime.2 In addition, one in 13 men are affected by subfertility or infertility conditions.3 Although some conditions, such as infertility, benign prostatic hyperplasia (BPH) and indolent prostate cancer primarily affect quality of life, other conditions, such as aggressive prostate cancer, are life-threatening and should be diagnosed early and treated appropriately (Box 1, Table 1).
In this Review, we focus on seminal plasma and its use as a biological fluid for the discovery of biomarkers of male reproductive system disorders, and as a clinical sample for noninvasive urogenital diagnostics. We believe that seminal plasma has great potential for both of these applications, as it contains components that are very specific to particular organs in the male urogenital tract and, therefore, differences in protein composition of semen might indicate an ongoing pathological process in a specific organ. This concept is best illustrated by the discovery of PSA as a marker of prostate diseases—PSA was originally discovered in, and isolated from, semen. PSA, which is the most common marker in use to identify prostate cancer, is found at much higher concentrations in semen than in blood serum. Similar to the story of PSA discovery,4 potential biomarkers might be present at much greater abundances in seminal plasma than in serum or urine; these biomarkers have the potential to be easily identified and quantified by comprehensive analytical techniques, such as mass spectrometry.
Several review articles published in 2013 summarized the molecular composition of seminal plasma and the functions of seminal plasma proteins,5,6,7 focusing mainly on aspects of reproductive biology. We focus on proteomics and mass spectrometry as major analytical techniques to identify novel protein biomarkers in seminal plasma, and provide examples of emerging biomarkers of male infertility and prostate cancer. In addition, we highlight the value of tissue-specific proteins for diagnostics and present approaches for the integration of multiple 'omics' techniques in the quest for novel biomarkers. Finally, we critically assess the place of seminal plasma in the clinical pathway for the diagnosis of disorders of the male reproductive system, as viewed from the urologist's perspective.
Role and composition of seminal plasma
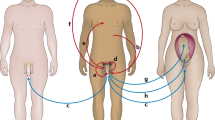
Seminal plasma is a biological fluid composed of secretions from glands in the male urogenital tract (Figure 1). During ejaculation, sperm passes through the ejaculatory ducts and combines with fluids from the glands to generate semen. Seminal plasma, which is the supernatant remaining after centrifugation and removal of cells and cell debris from the liquefied semen, constitutes >90% of semen.
Seminal plasma proteins arise from secretions from the seminal vesicles (∼65% of semen volume), prostate (∼25%), testes and epididymides (∼10%) and bulbourethral and periurethral glands (∼1%). Seminal vesicles contribute the greatest molecular content, including fructose, semenogelins, protein C-inhibitor and mucin 6. The prostate gland secretes a fluid consisting of citrate, lipids, prostatic acid phosphatase and proteolytic enzymes, such as kallikreins. Bulbourethral and numerous periurethral glands mostly secrete mucinous proteins. Secretions from testes and epididymides into seminal plasma include hundreds of proteins related to spermatogenesis and maturation of spermatozoa during epididymal transit. To identify low-abundance proteins in seminal plasma, fluids enriched with tissue-specific proteins can be collected by the methods indicated. Abbreviations: MESA, microsurgical epididymal sperm aspiration; RP, radical prostatectomy.
From a functional perspective, seminal plasma not only carries and provides nutrition for spermatozoa, but also modulates the function of spermatozoa and their interaction with the female genital tract, especially with components of the female immune system. Seminal plasma has important roles in the regulation of semen coagulation and liquefaction, sperm motility and fertilization.
The molecular composition of seminal plasma is diverse, ranging from lipids, glycans, inorganic ions and small molecule metabolites to biopolymers, such as cell-free DNA, RNA, microRNAs, peptides, proteins and oligosaccharides. Fructose, which is present at very high levels, along with lipids, are important sources of energy for spermatozoa.8 One of the most common inorganic ions found in seminal plasma is zinc, which is a cofactor or inhibitor for many proteolytic enzymes involved in the coagulation–liquefaction process.9 Copper and selenium in semen are crucial components of antioxidant enzymes and are essential for normal spermatogenesis.10 Cell-free DNA and various RNA species have been identified in seminal plasma, but the roles, origins and relative concentrations of such species remain largely unknown.11 MicroRNAs have been identified as potential biomarkers for spermatogenesis status, and further experiments are required to assess their usefulness for diagnostics.12 In addition, glycoproteins and glycans are now being assessed for their potential as biomarkers for different urogenital disorders.7
Among the individual glands, the seminal vesicles contribute the greatest molecular content into seminal plasma, notably cytokines, prostaglandins and fructose (Figure 1).13 The prostate glands secrete a fluid consisting of proteolytic enzymes, citrate and lipids.14,15,16 The secretions of the seminal vesicles and prostate are alkaline, which is important for the survival of sperm cells in the acidic vaginal environment.16 The alkaline environment of semen is maintained by basic polyamines, such as spermine, spermidine and putrescine. The bulbourethral glands secrete galactose, sialic acid and mucus that lubricates the semen, enabling more efficient sperm transfer.16
Despite being rich in components with potential diagnostic value, seminal plasma has rarely been assessed in the clinic. Among the few existing examples, measurement of fructose levels indicates abnormalities in seminal vesicles,17 and measurement of prostatic acid phosphatase reveals the presence of prostatic secretions in seminal plasma.18
The seminal plasma proteome
The development of seminal plasma proteomics
The first published studies on seminal plasma proteins date back to 1888 when propeptone, a mixture of products of proteolytic digestion, was detected in urine and was traced to contamination with semen.19 The electrophoretic separation of seminal plasma proteins was reported in 1942, and four protein components—albumin, α-globulin, β-globulin and γ-globulin—were identified.20,21 In the late 1970s, advances in electrophoretic separations resulted in the detection of 40 protein spots with 1D gel electrophoresis,22 followed by the observation of ∼200 protein spots using 2D gel electrophoresis.23 The implementation of soft ionization and mass spectrometry methods has enabled the identification of many more proteins and revealed the complexity of seminal plasma.24,25 In the past 3 years, the most prominent studies have used 2D liquid chromatography separations coupled to electrospray ionization and detection of mass spectra with Orbitrap™ (Thermo Fisher Scientific, USA) instruments.26,27,28,29 These studies have identified thousands of proteins in seminal plasma and tissues of the male reproductive system (Table 2). For example, our group identified nearly 3,200 proteins in total, which constitutes the largest library of seminal plasma proteins reported to date.26,30,31 In addition, as many as 7,346 proteins were identified in testicular tissue,28 and 10,369 were detected in a prostate cancer cell line,27 which might be close to the entire proteomes of these tissues.
Composition, complexity and selective analysis
Seminal plasma proteins arise from secretions from seminal vesicles (∼65% of semen volume), prostate (∼25%), testis and epididymis (∼10%) and bulbourethral and periurethral glands (∼1%).14,32,33 The proteome of seminal plasma is as complex as the blood plasma proteome. It contains large amounts of semenogelins, PSA and other secreted high-abundance proteins at a total concentration of 40–60 mg/ml,26,30,34,35,36 and has a dynamic range of around nine orders of magnitude, with the least abundant proteins being proinflammatory interleukins, which are present at 10 pg/ml.37 As with proteomic analysis of blood plasma, identification of low-abundance proteins in seminal plasma by mass spectrometry is challenging, owing to the wide dynamic range of protein concentrations. Particular obstacles are the high-abundance proteins expressed by the seminal vesicles and prostate. In blood plasma, the top 10 proteins represent ∼90% of the total protein concentration. Our preliminary estimates show that the top 10 seminal plasma proteins represent >80% of the total protein concentration, with semenogelins accounting for up to 30% (unpublished data).
Collection of fluids secreted by individual glands provides samples enriched with tissue-specific proteins (Figure 1). For example, expressed prostatic secretions can be obtained upon prostate massage, whereas epididymal and seminal vesicle fluids are collected by microsurgical epididymal sperm aspiration and seminal vesicle fluid aspiration guided by transrectal ultrasonography, respectively. Likewise, ejaculates from men who have undergone vasectomy are devoid of testicular and epididymal proteins, whereas ejaculates from men who have been treated by radical prostatectomy contain secretions exclusively from bulbourethral and periurethral glands. To deplete high-abundance proteins or enrich low-abundance proteins prior to mass spectrometry, a variety of analytical separation techniques are used (Figure 2), such as immunodepletion, size-exclusion of high-abundance peptides of semenogelins, combinatorial peptide ligand libraries,38,39 multidimensional chromatographic separations,30 cell line secretome analysis and N-glycoprotein enrichment or immunoenrichment.40
A variety of analytical separation techniques are used to deplete high-abundance proteins or enrich low-abundance proteins prior to identification by mass spectrometry. Abbreviation: LC-MS, liquid chromatography-mass spectrometry.
Physiological roles of seminal plasma proteins
Proteomic and functional studies have shed light on the physiological roles of seminal plasma proteins. They are involved in a range of molecular processes, including maintenance of an alkaline milieu, sperm nutrition and transport, coagulation and liquefaction of the ejaculate, augmentation and inhibition of sperm motility, augmentation of the immune response, interaction with the zona pellucida, modulation of the acrosome reaction, degradation of the extracellular matrix and fusion with the oocyte membrane. Regulation of the immune response is a critical function of seminal plasma and is provided by immunosuppressive cytokines, with the level of TGF-β reaching 150–200 ng/ml,41 which far exceeds its concentration in any other body fluid, including blood plasma.42
The functional roles of seminal plasma proteins are related to the function of each gland. The most abundant proteins in secretions from seminal vesicles include semenogelins, fibronectin, protein C-inhibitor, mucin 6 and prolactin-inducible protein.43 Products of proteolytic cleavage of semenogelins inhibit sperm motility and provide antibacterial action. Secretions from the epididymis include clusterin, glutathione peroxidase 5, prostaglandin D2 synthase (PTGDS) and human epididymal protein E4 (HE4). PTGDS function in seminal plasma includes transport of retinoids,44 and HE4 protein serves as a pan–serine protease inhibitor.45 Periurethral and bulbourethral glands mainly produce colloid secretions and mucinous proteins that neutralize the residual acidity and protect the epithelium and spermatozoa against urine.15 The prostate secretes kallikreins, prostatic acid phosphatase, zinc-α2-glycoprotein and β-microseminoprotein. These proteins are involved in the proteolytic cleavage of semenogelins,46 hydrolysis of phosphomonoesters,47 lipid mobilization48 and protection against fungal infections,49 respectively. The concentration of PSA (kallikrein 3) is 300,000-fold higher in seminal plasma than in blood serum (1.2 mg/ml50 versus 4 ng/ml51).
In the search for biomarkers of male reproductive system disorders, different functional categories of seminal plasma proteins can be considered. For example, proteins directly related to spermatogenesis and fertilization could perform well as biomarkers of male infertility,52 inflammatory proteins might be potential biomarkers of prostatitis,31 and cancer-associated proteins could be biomarkers of prostate and testicular cancers.
Nonsecreted proteins of seminal plasma
In addition to secreted proteins, seminal plasma contains intracellular and membrane proteins originating from spermatozoa, immature germ cells, leukocytes, exfoliated prostate cells,53 epididymis,54 seminal vesicle cells and epithelial cells. For example, TEX101, an abundant seminal plasma protein with monospecific expression in germ cells, is shed from the surface of sperm cells during their passage through the epididymis,40,55 and T cells contribute to the presence of CD4 protein.56 Common blood plasma proteins identified in seminal plasma, such as albumin, transferrin, complement factors and immunoglobulins, derive from intercellular fluids.57
Although the majority of seminal plasma proteins are found in the soluble protein fraction, nearly 3% are identified in microvesicles, such as prostasomes and epididymosomes.58 In the search for novel proteins that might emerge as markers of prostate cancer, BPH and prostatitis, studies have profiled the proteome of prostasomes,59,60,61 and identified as many as 416 unique proteins (Table 2).60
Tissue-specific proteins as biomarkers
Seminal plasma proteins might be a rich source of biomarkers for the diagnosis of male reproductive system disorders, as the concentration of many of the proteins is high, and many are tissue specific,62 enabling the search for biomarkers of diseases of specific organs. Abnormal changes in the concentration of tissue-specific proteins can indicate a progressing pathological process in the specific organ or tissue.
Blood–tissue barriers
Glands and tissues of the male reproductive system have stringent blood–tissue barriers, such as blood–testis, blood–prostate and blood–epididymis barriers.63,64 As a result, tissue-specific prostatic, testicular and epididymal proteins are normally not found at significant levels in the blood plasma of healthy individuals, but could increase sharply in pathological processes that result in tissue destruction. An example is the human epididymal protein E4 (HE4), which normally has strong expression in the epididymis and weak expression in other organs. In 2008, HE4 was cleared by the FDA as a biomarker for monitoring women with epithelial ovarian cancer.65 In agreement with the cancer/testis antigen hypothesis, proteins that are normally expressed specifically in prostatic, testicular and epididymal tissues are identified in the systemic circulation as a result of tumour development in distant organs.66,67 Owing to normally stringent blood–tissue barriers and local sequestration, seminal plasma proteins leaking into blood can result in strong immune responses.68 Destruction of male reproductive system tissues or cancer development in distant organs could, therefore, lead to the production of autoantibodies against seminal plasma proteins found in blood, and the presence of these autoantibodies could also be used for diagnostic applications.66,69 Furthermore, since prostate-expressed proteins leak into blood plasma only after the destruction of blood–tissue barriers (prostate cancer diagnosed with serum PSA >4 ng/ml at the clinical stage T1c already has an average volume of 1.8 cm3),70 we believe that analysis of seminal plasma proteins can facilitate early diagnosis of prostate cancer at the clinical stages T1a and T1b.
Identification of tissue-specific proteins
Tissue specificity, a valuable filtering criterion for biomarker discovery pipelines, can be identified by the use of expression databases.71 Gene expression databases, such as BioGPS,72 and the Cancer Genetics database,73 contain mRNA expression data for multiple human tissues. Protein expression databases, such as the Human Protein Atlas,74 contain protein data based on immunohistochemistry profiling of human tissues. The Human Protein Atlas promises to provide the first draft of the human proteome in 2015, with the most recent update (version 12) containing protein expression profiles of 16,621 genes.
A search for tissue-specific transcripts and proteins in the Cancer Genetics database73 and Human Protein Atlas74 reveals 586 testis-specific mRNAs and 127 proteins, respectively (Figure 3). Interestingly, the number of testis-specific transcripts and proteins substantially exceeds the number of tissue-specific transcripts and proteins in any other human tissue. Some promising biomarkers for male infertility and prostate cancer include ECM1,40 TEX101,40 LDHC,52 TKTL138 and ACPP75 proteins.
a | The Cancer Genetics database73 was used to identify tissue-specific mRNA. Genes with restricted expression (10-fold higher than the median expression in 42 human tissues) in five or fewer tissues were considered tissue-specific. b | The Human Protein Atlas (version 11.0)74 was used to identify tissue-specific proteins. Proteins with restricted expression (strong, moderate and weak) in five or fewer out of 82 human tissues or cells were considered tissue-specific. The number of testis-specific transcripts and proteins substantially exceeds the number of tissue-specific transcripts and proteins in other human tissues.
Differential diagnosis of azoospermia
Semen analysis for the presence of spermatozoa in the seminal fluid is a common test when male infertility is suspected. Azoospermia, the most severe form of male infertility, is associated with absence of sperm in the semen, and has two major forms—obstructive azoospermia and nonobstructive azoospermia. Obstructive azoospermia, caused by physical obstruction in the male reproductive tract, results from vasal or epididymal pathologies and is responsible for ∼40% of azoospermia cases.76 Clinical outcomes of obstructive azoospermia are identical to those of vasectomy, and result in male sterilization. Nonobstructive azoospermia, also referred to as testicular failure, is classified into subtypes of Sertoli-cell-only syndrome, maturation arrest and hypospermatogenesis,77 based on histopathological analysis of the biopsied testicular tissue.
Testicular histology with surgical exploration of the genital tract is currently the only reliable method for differential diagnosis of azoospermia.78 Results of diagnostic testicular biopsy, however, might not accurately reflect the histopathology of nonobstructive azoospermia, owing to the spatial distribution of spermatogenesis in the testis. Noninvasive differential diagnosis of azoospermia forms and subtypes is, therefore, an unmet need in the management of male infertility. In patients with nonobstructive azoospermia, a good diagnostic test should reveal the severity of testicular failure, provide more accurate diagnosis of histopathological subtypes, predict the success of testicular sperm extraction and facilitate better planning for assisted reproduction. In men with normal spermatogenesis who have undergone vasectomy or vasovasostomy, the diagnostic test should show the efficiency of vas deferens severance or ligation. Finally, in patients with cancer who are treated with radiation and chemotherapy, the diagnostic test should reveal the decline or recovery of spermatogenesis.
Many studies have evaluated the prediction of azoospermia forms and subtypes using testicular volume or blood biomarkers, such as follicle-stimulating hormone, inhibin B and anti-Müllerian hormone.79,80,81 However, these markers have poor specificity and sensitivity, and proteins measured in proximal fluids, such as seminal plasma, might have better predictive value.52,62 For example, the seminal plasma protein PTGDS has been proposed to be a marker of obstructive azoospermia or vasectomy.82 In addition, an immunodiagnostic home test for ACRV1 protein in semen is commercially available and can estimate the number of spermatozoa (SpermCheck® [ContraVac, USA] Fertility test) or provide evidence of vasectomy success (SpermCheck® Vasectomy test).83,84 However, given that ACRV1 is a protein associated with the inner acrosomal membrane and is only fully released from spermatozoa upon detergent-mediated cell lysis, the utility of ACRV1 as a seminal plasma biomarker for differential diagnosis of azoospermia remains unknown. Proteomic studies have also identified and verified, in small sets of samples, PARK7 (DJ-1) protein as a biomarker of asthenozoospermia (reduced sperm motility),36 and TKTL1, LDHC and PGK2 proteins as biomarkers of obstructive azoospermia and vasectomy.38
The possibility of using seminal plasma markers to differentiate obstructive azoospermia from nonobstructive azoospermia, and to identify the different categories of nonobstructive azoospermia, has been proposed.38,52 Using tandem mass spectrometry, our group identified >2,000 proteins in seminal plasma of men with normal spermatogenesis, men with nonobstructive azoospermia and men who had undergone vasectomy, and suggested a list of 79 biomarker candidates.26,30 In our follow-up work, biomarker candidates were verified and validated and a panel of 18 biomarkers for differential diagnosis of azoospermia was proposed.40,52 Two proteins, the epididymis-expressed ECM1 and testis-expressed TEX101, emerged as biomarkers for differentiation of azoospermia forms, each with >95% specificity and sensitivity. Interestingly, seminal plasma levels of TEX101 were also able to differentiate between nonobstructive azoospermia histopathological subtypes.40 The discriminatory ability of TEX101 protein originates from its specific expression in germ cells, but not in any other human cells or tissues, as revealed by the Human Protein Atlas.74 As a result, a simple two-marker algorithm for highly sensitive and specific differential diagnosis of azoospermia forms and nonobstructive azoospermia histopathological subtypes by a noninvasive test was proposed by our group in 2013 (Figure 4).40 Noninvasive diagnostic tests with ECM1 and TEX101 proteins have the potential to eliminate diagnostic testicular biopsies, improve the confidence of azoospermia diagnosis and facilitate prediction of the outcome of sperm retrieval procedures used for assisted reproduction. Our study also demonstrated that germ-cell-specific proteins perform well as biomarkers of azoospermia. Further studies of testis-specific proteins could provide a seminal plasma panel of markers to assess individual stages of spermatogenesis (development of spermatogonia into mature spermatozoa).
When azoospermia is diagnosed by semen analysis, very low seminal plasma levels of ECM1 (<2.3 µg/ml) and TEX101 (<5 ng/ml) proteins suggest obstruction of vas deferens, but a high seminal plasma level of ECM1 (≥2.3 µg/ml) suggests nonobstructive azoospermia. TEX101 protein also distinguishes between nonobstructive azoospermia subtypes of Sertoli-cell-only syndrome (<5 ng/ml) and hypospermatogenesis or maturation arrest (5–120 ng/ml). Men with obstructive azoospermia have high chances of successful sperm retrieval by TESE, while for men with Sertoli-cell-only syndrome, sperm retrieval is unlikely, and TESE could be avoided. Abbreviation: TESE, testicular sperm extraction. From Drabovich, A. P. et al. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci. Transl. Med. 5, 212ra160 (2013).40 Reprinted with permission from AAAS.
Prostate cancer biomarkers
The field of prostate cancer diagnostics was revolutionized by the discovery of PSA. Since the introduction of the serum PSA test, prostate cancer diagnosis has become more frequent.85,86 However, the PSA biomarker has a number of limitations, including lack of specificity and prognostic significance, and an inability to differentiate indolent from aggressive disease.87,88 PSA expression is prostate-tissue-specific but not prostate-cancer-specific, as PSA is elevated in other nonmalignant pathologies of the prostate, including BPH and prostatitis.87,88 As a result of these limitations, 50–75% of patients who present with an elevated PSA level have a negative prostate biopsy result.89 With these limitations of PSA testing, a number of studies have aimed to identify novel prostate cancer biomarkers using genomic, epigenetic and proteomic methods applied to cell lines, tissues, serum, urine and proximal fluids, such as expressed prostatic secretions.73,90,91,92,93 However, the markers identified by these studies are still at the preclinical stage, or have failed to improve on PSA. Thus, a clinical need exists for the identification of additional biomarkers that can be used separately to, or in conjunction with PSA, to address these limitations. Analysis of markers in seminal plasma and expressed prostatic secretions could become a viable alternative or complementary approach for prostate cancer diagnosis and prognosis and might provide higher specificity for detecting prostate cancer and for differentiating indolent and aggressive forms.75 For example, PROS1 protein was evaluated as a seminal-plasma-based biomarker of aggressive prostate cancer and showed some promise (area under the curve = 0.87), albeit with a small set of samples.90 Likewise, MME and TIMP1 proteins were measured in a small set of expressed prostatic secretions and differentiated moderately well between extracapsular (high-grade) and organ-confined (low-grade) prostate cancer.75
An example of how seminal-plasma-based prostate cancer markers could potentially be useful is provided by ETS gene fusions, specifically the TMPRSS2–ERG and TMPRSS2-ETV1 fusions, which have been identified in >50% of prostate cancer cases studied.94 Although such a fusion might not be a strong individual biomarker, it is an early event during prostate cancer development and could represent a unique lineage of prostate cancer.95 Tumours containing such fusions might have their own unique biomarker profiles, so future work, including seminal plasma analysis, could focus on identifying unique markers in patients with and without the gene fusions. In conjunction with fusion status and other pathological features of prostate cancer (including Gleason score and pathological stage), seminal-plasma-based markers could provide diagnostic and prognostic power to further discriminate indolent from aggressive prostate cancers, a key unmet clinical need.
Integration of 'omics' data
Advances in genomics, epigenomics, transcriptomics and proteomics have resulted in the accumulation of immense amounts of data on disease-related genes, mRNA transcripts and proteins. Translation of these data into clinical diagnostics has not yet been fully achieved. Little is yet known about which genomic, epigenetic and transcriptomic alterations can be measured at the protein level in biological fluids.
After the sequencing of the human genome,96 large-scale international projects initiated detailed investigations into elements of the genome, including elucidation of disease-relevant genes,97 single nucleotide polymorphisms (SNPs)98,99 and functional elements of the genome, such as regions of differential transcription, chromatin structure, transcription factor associations and histone modifications.100 Several cancer genome projects have provided a comprehensive description of genomic, transcriptomic and epigenomic changes in multiple tumour types and subtypes.101,102 Since 2008, genome-wide association studies have linked SNPs with the risk of male infertility,103,104,105 prostate106,107,108 and testicular cancers.109,110 Information on differential transcriptomic changes and mRNA alternative splicing under different biological conditions has also been included in publicly available databases.111,112 Finally, the Human Proteome Project has been launched as a global effort to catalogue the abundance, subcellular localization and function of all human proteins in health and disease.113
Disease-specific genomic alterations can be translated into proteins directly, through altered transcription, alternative splicing, translation and post-translational modifications (qualitative and quantitative changes),114,115,116 as well as indirectly, through epigenetic regulation, noncoding RNA regulation and altered signal transduction.117,118 Genomic alterations that can be used for diagnostics at the proteome level include missense SNPs and somatic mutations, protein-coding gene fusions, copy number gains and losses, disease-specific alternative splicing, disease-specific protein isoforms or post-translational modifications, and alterations in protein expression due to abnormal DNA methylation, histone modification, nucleosome remodeling and microRNA regulation.119 Notably, qualitative alterations resulting in amino acid substitutions in the protein sequence are relatively rare, owing to the degeneracy of the genetic code, but they can still be evaluated as biomarkers at the protein level.114,120
Integration of disease-specific genomic, epigenomic, transcriptomic and proteomic alterations provides a comprehensive approach to discover protein biomarkers in seminal plasma (Figure 5). Since 2010, several studies have integrated multiple 'omics' approaches to identify male infertility and prostate cancer biomarker candidates,38,117 or genes involved in disease progression.73,121 Integration of 'omics' approaches might reveal not only individual biomarkers, but also biomarker panels and molecular pathways altered in disease, such as metabolic pathways in cancer.122,123 The diagnostic performance of biomarker panels and protein networks to detect highly heterogeneous diseases, such as cancer, could be far superior to the performance of single biomarkers.
Disease-specific genomic, epigenomic, transcriptomic and proteomic alterations measured in tissues, cell lines and proximal fluids, such as expressed prostatic secretions (EPS), provide a comprehensive approach to the discovery of protein biomarkers in seminal plasma. Disease-specific genomic alterations are translated into proteins directly through transcription and translation as well as indirectly through epigenetic regulation, noncoding RNA regulation and altered signal transduction. For example, genomic alterations that can be used for diagnostics at the proteome level include missense SNPs and somatic mutations, protein-coding gene fusions and disease-specific alternative splicing forms or protein isoforms.
Seminal plasma in the clinical arena
Discussion of the potential of seminal plasma biomarkers is not complete without considering how they can complement current diagnostic tools used in urology clinics.124 Common clinical procedures to diagnose diseases of the male reproductive system include physical examination, imaging, surgical techniques and blood, urine and semen-based diagnostic tests. Noninvasive imaging techniques and tests using blood and urine are the preferred diagnostic tools from the patient's perspective. Thus, seminal-plasma-based biomarkers will enter the clinic only if they outperform or complement available imaging techniques and blood-based tests, help to avoid invasive procedures (such as biopsies) and provide diagnostic information that cannot be obtained by alternative methods.
Modern noninvasive imaging techniques used in urology clinics include MRI125 and PET.126 Transrectal ultrasonography is mainly used to guide needle biopsies, but is not employed alone as a diagnostic tool, owing to its relatively low resolution. The high cost of cyclotrons, use of radionuclides and exposure to ionizing radiation limit the widespread use of PET. MRI remains the most promising imaging technique in terms of noninvasiveness and safety, but its routine use is restrained by high costs, duration of analysis and insufficient resolution. Although most urological disorders do not require emergency diagnosis and intervention, a few conditions, such as acute prostatic inflammation, might need to be quickly confirmed by diagnostic tests. Finally, advanced imaging techniques are not necessarily available in small medical centres and are not quickly accessible in remote areas. These limitations of imaging techniques create a niche for molecular biomarker-based tests that could facilitate prompt decision-making at low cost and high accessibility.
Although seminal plasma has great potential as a clinical diagnostic fluid, it is possible that it might not be readily accepted, owing to ethical or religious considerations,127 or potential difficulties obtaining semen from elderly men who are often most at risk of reproductive system disorders. However, our discussions with patients suggest that seminal-plasma-based analysis for biomarkers of prostate cancer aggressiveness would be preferable to prostate biopsy. Stringent blood–tissue barriers (giving independence from the systemic circulation) and the intimate physical and functional association with the sites of urogenital diseases make it highly likely that seminal plasma will find its niche for early cancer diagnosis and diagnosis of other diseases of the male reproductive system.
Conclusions
Disorders of the male reproductive system affect the quality of men's lives, especially in the ageing population. Proper treatment of such disorders is limited by the absence of accurate diagnostic methods. Although medical imaging and blood-based tests are currently the preferred diagnostic methods, they are far from ideal. We believe that seminal plasma has potential as a biological fluid for the discovery of novel biomarkers, and as a clinical sample for noninvasive urogenital diagnostics. Seminal plasma biomarker discovery will be greatly aided by the integration of ever more powerful 'omics' technologies. Noninvasive differential diagnosis of male infertility and detection of aggressive prostate cancer could emerge as the most beneficial uses of seminal plasma diagnostics.
Review criteria
The PubMed database was searched for full-text English-language articles published from 1942 to 2013 using the search terms “seminal plasma proteins”, “semen proteins”, “seminal fluid proteins”, “proteomics and biomarker discovery”, “proteomics and mass spectrometry”, “male reproductive system disorders”, “prostate cancer biomarkers” and “male infertility biomarkers” in various combinations. The majority of articles that we included were published after January 2005, while some older articles were used to address the history of seminal plasma analysis. The reference lists of selected articles were searched for further relevant publications.
References
Krieger, J. N., Ross, S. O. & Riley, D. E. Chronic prostatitis: epidemiology and role of infection. Urology 60, 8–12 (2002).
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30 (2013).
Mosher, W. D. Reproductive impairments in the United States, 1965–1982 Demography 22, 415–430 (1985).
Rao, A. R., Motiwala, H. G. & Karim, O. M. The discovery of prostate-specific antigen. BJU Int. 101, 5–10 (2008).
Laflamme, B. A. & Wolfner, M. F. Identification and function of proteolysis regulators in seminal fluid. Mol. Reprod. Dev. 80, 80–101 (2013).
Upadhyay, R. D. et al. Proteomics in reproductive biology: beacon for unraveling the molecular complexities. Biochim. Biophys. Acta 1834, 8–15 (2013).
Ferens-Sieczkowska, M., Kowalska, B. & Kratz, E. M. Seminal plasma glycoproteins in male infertility and prostate diseases: is there a chance for glyco-biomarkers? Biomarkers 18, 10–22 (2013).
Lenzi, A., Picardo, M., Gandini, L. & Dondero, F. Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 2, 246–256 (1996).
Yoshida, K. et al. Physiological roles of semenogelin I and zinc in sperm motility and semen coagulation on ejaculation in humans. Mol. Hum. Reprod. 14, 151–156 (2008).
Camejo, M. I. et al. Selenium, copper and zinc in seminal plasma of men with varicocele, relationship with seminal parameters. Biol. Trace Elem. Res. 143, 1247–1254 (2011).
Li, H. G., Huang, S. Y., Zhou, H., Liao, A. H. & Xiong, C. L. Quick recovery and characterization of cell-free DNA in seminal plasma of normozoospermia and azoospermia: implications for non-invasive genetic utilities. Asian J. Androl. 11, 703–709 (2009).
Wu, W. et al. Seminal plasma microRNAs: potential biomarkers for spermatogenesis status. Mol. Hum. Reprod. 18, 489–497 (2012).
Gonzales, G. F. Function of seminal vesicles and their role on male fertility. Asian J. Androl. 3, 251–258 (2001).
Mann, T. The biochemistry of semen (eds Peters, R. & Young, F. G.) (Methuen, Wiley, 1954).
Mann, T. & Lutwak-Mann, C. Male reproductive function and semen (Springer-Verlag, 1981).
Mann, T. Secretory function of the prostate, seminal vesicle and other male accessory organs of reproduction. J. Reprod. Fertil. 37, 179–188 (1974).
Gonzales, G. F. & Villena, A. True corrected seminal fructose level: a better marker of the function of seminal vesicles in infertile men. Int. J. Androl. 24, 255–260 (2001).
Drake, R. R. et al. Clinical collection and protein properties of expressed prostatic secretions as a source for biomarkers of prostatic disease. J. Proteomics 72, 907–917 (2009).
Posner, C. Berl. Klin. Wochenschr. 25, 416 (1888).
Gray, S. & Huggins, C. Electrophoretic analysis of human semen. Proc. Soc. Exp. Biol. Med. 50, 351–353 (1942).
Ross, V., Moore, D. H. & Miller, E. G. Proteins of human seminal plasma. J. Biol. Chem. 144, 667–677 (1942).
Sensabaugh, G. F. Isolation and characterization of a semen-specific protein from human seminal plasma: a potential new marker for semen identification. J. Forensic Sci. 23, 106–115 (1978).
Edwards, J. J., Tollaksen, S. L. & Anderson, N. G. Proteins of human semen. I. Two-dimensional mapping of human seminal fluid. Clin. Chem. 27, 1335–1340 (1981).
Fung, K. Y., Glode, L. M., Green, S. & Duncan, M. W. A comprehensive characterization of the peptide and protein constituents of human seminal fluid. Prostate 61, 171–181 (2004).
Starita-Geribaldi, M. et al. Development of narrow immobilized pH gradients covering one pH unit for human seminal plasma proteomic analysis. Proteomics 3, 1611–1619 (2003).
Batruch, I. et al. Analysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertility. J. Proteome Res. 11, 1503–1511 (2012).
Geiger, T., Wehner, A., Schaab, C., Cox, J. & Mann, M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol. Cell. Proteomics 11, M111.014050 (2012).
Liu, M. et al. Scanning of novel cancer/testis proteins by human testis proteomic analysis. Proteomics 13, 1200–1210 (2013).
Wang, G. et al. In-depth proteomic analysis of the human sperm reveals complex protein compositions. J. Proteomics 79, 114–122 (2013).
Batruch, I. et al. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J. Proteome Res. 10, 941–953 (2011).
Kagedan, D. et al. Characterization of the seminal plasma proteome in men with prostatitis by mass spectrometry. Clin. Proteomics 9, 2 (2012).
Owen, D. H. & Katz, D. F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 26, 459–469 (2005).
Robert, M. & Gagnon, C. Sperm motility inhibitor from human seminal plasma: presence of a precursor molecule in seminal vesicle fluid and its molecular processing after ejaculation. Int. J. Androl. 17, 232–240 (1994).
Pilch, B. & Mann, M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 7, R40 (2006).
Milardi, D. et al. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil. Steril. 97, 67–73. e1 (2012).
Wang, J. et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J. Androl. 11, 484–491 (2009).
Penna, G. et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 51, 524–533 (2007).
Rolland, A. D. et al. Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum. Reprod. 28, 199–209 (2012).
Drabovich, A. P. & Diamandis, E. P. Combinatorial peptide libraries facilitate development of multiple reaction monitoring assays for low-abundance proteins. J. Proteome Res. 9, 1236–1245 (2010).
Drabovich, A. P. et al. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci. Transl. Med. 5, 212ra160 (2013).
Robertson, S. A., Ingman, W. V., O'Leary, S., Sharkey, D. J. & Tremellen, K. P. Transforming growth factor β--a mediator of immune deviation in seminal plasma. J. Reprod. Immunol. 57, 109–128 (2002).
Politch, J. A., Tucker, L., Bowman, F. P. & Anderson, D. J. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum. Reprod. 22, 2928–2935 (2007).
Duncan, M. W. & Thompson, H. S. Proteomics of semen and its constituents. Proteomics Clin. Appl. 1, 861–875 (2007).
Tanaka, T. et al. Lipocalin-type prostaglandin D synthase (β-trace) is a newly recognized type of retinoid transporter. J. Biol. Chem. 272, 15789–15795 (1997).
Kirchhoff, C., Habben, I., Ivell, R. & Krull, N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol. Reprod. 45, 350–357 (1991).
Lilja, H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J. Clin. Invest. 76, 1899–1903 (1985).
Tanaka, M. et al. Prostatic acid phosphatase degrades lysophosphatidic acid in seminal plasma. FEBS Lett. 571, 197–204 (2004).
Hirai, K., Hussey, H. J., Barber, M. D., Price, S. A. & Tisdale, M. J. Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res. 58, 2359–2365 (1998).
Edstrom Hagerwall, A. M. et al. β-Microseminoprotein endows post coital seminal plasma with potent candidacidal activity by a calcium- and pH-dependent mechanism. PLoS Pathog. 8, e1002625 (2012).
Wang, T. J., Rittenhouse, H. G., Wolfert, R. L., Lynne, C. M. & Brackett, N. L. PSA concentrations in seminal plasma. Clin. Chem. 44, 895–896 (1998).
Catalona, W. J. et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N. Engl. J. Med. 324, 1156–1161 (1991).
Drabovich, A. P., Jarvi, K. & Diamandis, E. P. Verification of male infertility biomarkers in seminal plasma by multiplex selected reaction monitoring assay. Mol. Cell. Proteomics 10, M110.004127 (2011).
Andrade-Rocha, F. T. Assessment of exfoliated prostate cells in semen: relationship with the secretory function of the prostate. Am. J. Clin. Pathol. 128, 788–793 (2007).
Andrade-Rocha, F. T. Assessment of increased desquamation of epididymal epithelial cells in semen of men as a predictor of acute epididymitis. Urol. J. 8, 320–322 (2011).
Fujihara, Y. et al. Expression of TEX101, regulated by ACE, is essential for the production of fertile mouse spermatozoa. Proc. Natl Acad. Sci. USA 110, 8111–8116 (2013).
Olivier, A. J. et al. Isolation and characterization of T cells from semen. J. Immunol. Methods 375, 223–231 (2012).
Martinez-Prado, E. & Camejo Bermudez, M. I. Expression of IL-6, IL-8, TNF-α, IL-10, HSP-60, anti-HSP-60 antibodies, and anti-sperm antibodies, in semen of men with leukocytes and/or bacteria. Am. J. Reprod. Immunol. 63, 233–243 (2010).
Ronquist, G. & Brody, I. The prostasome: its secretion and function in man. Biochim. Biophys. Acta 822, 203–218 (1985).
Utleg, A. G. et al. Proteomic analysis of human prostasomes. Prostate 56, 150–161 (2003).
Sandvig, K. & Llorente, A. Proteomic analysis of microvesicles released by the human prostate cancer cell line PC-3. Mol. Cell. Proteomics 11, M111.012914 (2012).
Thimon, V., Frenette, G., Saez, F., Thabet, M. & Sullivan, R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum. Reprod. 23, 1698–1707 (2008).
Diamandis, E. P. et al. Seminal plasma biochemical markers and their association with semen analysis findings. Urology 53, 596–603 (1999).
Cheng, C. Y. & Mruk, D. D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 64, 16–64 (2012).
Mital, P., Hinton, B. T. & Dufour, J. M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod. 84, 851–858 (2011).
Anastasi, E. et al. HE4: a new potential early biomarker for the recurrence of ovarian cancer. Tumour Biol. 31, 113–119 (2010).
Chen, Y. T. et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl Acad. Sci. USA 94, 1914–1918 (1997).
Simpson, A. J., Caballero, O. L., Jungbluth, A., Chen, Y. T. & Old, L. J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 5, 615–625 (2005).
Fijak, M. & Meinhardt, A. The testis in immune privilege. Immunol. Rev. 213, 66–81 (2006).
Chen, Y. T. et al. Identification of multiple cancer/testis antigens by allogeneic antibody screening of a melanoma cell line library. Proc. Natl Acad. Sci. USA 95, 6919–6923 (1998).
Krumholtz, J. S. et al. Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology 60, 469–473 (2002).
Prassas, I., Chrystoja, C. C., Makawita, S. & Diamandis, E. P. Bioinformatic identification of proteins with tissue-specific expression for biomarker discovery. BMC Med. 10, 39 (2012).
Wu, C., Macleod, I. & Su, A. I. BioGPS and MyGene.info: organizing online, gene-centric information. Nucleic Acids Res. 41, D561–D565 (2013).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Fagerberg, L. et al. Contribution of antibody-based protein profiling to the human Chromosome-centric Proteome Project (C-HPP). J. Proteome Res. 12, 2439–2448 (2013).
Kim, Y. et al. Identification of differentially expressed proteins in direct expressed prostatic secretions of men with organ-confined versus extracapsular prostate cancer. Mol. Cell. Proteomics 11, 1870–1884 (2012).
Jarow, J. P., Espeland, M. A. & Lipshultz, L. I. Evaluation of the azoospermic patient. J. Urol. 142, 62–65 (1989).
McLachlan, R. I., Rajpert-De Meyts, E., Hoei-Hansen, C. E., de Kretser, D. M. & Skakkebaek, N. E. Histological evaluation of the human testis--approaches to optimizing the clinical value of the assessment: mini review. Hum. Reprod. 22, 2–16 (2007).
Dohle, G. R., Elzanaty, S. & van Casteren, N. J. Testicular biopsy: clinical practice and interpretation. Asian J. Androl. 14, 88–93 (2012).
Carpi, A., Sabanegh, E. & Mechanick, J. Controversies in the management of nonobstructive azoospermia. Fertil. Steril. 91, 963–970 (2009).
Muttukrishna, S. et al. Serum anti-Mullerian hormone and inhibin B in disorders of spermatogenesis. Fertil. Steril. 88, 516–518 (2007).
Tsametis, C. et al. Dynamic endocrine test of inhibin B and anti-Mullerian hormone in men with non-obstructive azoospermia. Gynecol. Endocrinol. 27, 661–665 (2011).
Heshmat, S. M. et al. Seminal plasma lipocalin-type prostaglandin D synthase: a potential new marker for the diagnosis of obstructive azoospermia. J. Urol. 179, 1077–1080 (2008).
Coppola, M. A. et al. SpermCheck Fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum. Reprod. 25, 853–861 (2010).
Klotz, K. L. et al. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J. Urol. 180, 2569–2576 (2008).
Barry, M. J. Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N. Engl. J. Med. 344, 1373–1377 (2001).
Heijnsdijk, E. A. et al. Quality-of-life effects of prostate-specific antigen screening. N. Engl. J. Med. 367, 595–605 (2012).
Diamandis, E. P. Prostate-specific antigen: its usefulness in clinical medicine. Trends Endocrinol. Metab. 9, 310–316 (1998).
Lopez-Otin, C. & Diamandis, E. P. Breast and prostate cancer: an analysis of common epidemiological, genetic, and biochemical features. Endocr. Rev. 19, 365–396 (1998).
McNaughton-Collins, M. F. & Barry, M. J. One man at a time--resolving the PSA controversy. N. Engl. J. Med. 365, 1951–1953 (2011).
Saraon, P. et al. Proteomic profiling of androgen-independent prostate cancer cell lines reveals a role for protein S during the development of high grade and castration-resistant prostate cancer. J. Biol. Chem. 287, 34019–34031 (2012).
Grasso, C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Khan, A. P. et al. Quantitative proteomic profiling of prostate cancer reveals a role for miR-128 in prostate cancer. Mol. Cell. Proteomics 9, 298–312 (2010).
Kim, Y. J. et al. EFEMP1 as a novel DNA methylation marker for prostate cancer: array-based DNA methylation and expression profiling. Clin. Cancer Res. 17, 4523–4530 (2011).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Salami, S. S. et al. Combining urinary detection of TMPRSS2:ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol. Oncol. 31, 566–571 (2013).
Venter, J. C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Stenson, P. D. et al. The Human Gene Mutation Database: 2008 update. Genome Med. 1, 13 (2009).
Abecasis, G. R. et al. An integrated map of genetic variation from 1, 092 human genomes. Nature 491, 56–65 (2012).
Frazer, K. A. et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
ENCODE Project Consortium et al. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Koboldt, D. C. et al. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Muzny, D. M. et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Kosova, G., Scott, N. M., Niederberger, C., Prins, G. S. & Ober, C. Genome-wide association study identifies candidate genes for male fertility traits in humans. Am. J. Hum. Genet. 90, 950–961 (2012).
Aston, K. I., Krausz, C., Laface, I., Ruiz-Castane, E. & Carrell, D. T. Evaluation of 172 candidate polymorphisms for association with oligozoospermia or azoospermia in a large cohort of men of European descent. Hum. Reprod. 25, 1383–1397 (2010).
Avenarius, M. R. et al. Human male infertility caused by mutations in the CATSPER1 channel protein. Am. J. Hum. Genet. 84, 505–510 (2009).
Thomas, G. et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat. Genet. 40, 310–315 (2008).
Eeles, R. A. et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat. Genet. 41, 1116–1121 (2009).
Kote-Jarai, Z. et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat. Genet. 43, 785–791 (2011).
Ruark, E. et al. Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nat. Genet. 45, 686–689 (2013).
Chung, C. C. et al. Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nat. Genet. 45, 680–685 (2013).
Barrett, T. et al. NCBI GEO: archive for functional genomics data sets--10 years on. Nucleic Acids Res. 39, D1005–D1010 (2011).
Kapushesky, M. et al. Gene Expression Atlas update--a value-added database of microarray and sequencing-based functional genomics experiments. Nucleic Acids Res. 40, D1077–D1081 (2012).
Legrain, P. et al. The human proteome project: current state and future direction. Mol. Cell. Proteomics 10, M111.009993 (2011).
Wang, Q. et al. Mutant proteins as cancer-specific biomarkers. Proc. Natl Acad. Sci. USA 108, 2444–2449 (2011).
Kuzmanov, U., Jiang, N., Smith, C. R., Soosaipillai, A. & Diamandis, E. P. Differential N-glycosylation of kallikrein 6 derived from ovarian cancer cells or the central nervous system. Mol. Cell. Proteomics 8, 791–798 (2009).
He, J. et al. Antibody-independent targeted quantification of TMPRSS2-ERG fusion protein products in prostate cancer. Mol. Oncol. http://dx.doi.org/10.1016/j.molonc.2014.02.004.
Cima, I. et al. Cancer genetics-guided discovery of serum biomarker signatures for diagnosis and prognosis of prostate cancer. Proc. Natl Acad. Sci. USA 108, 3342–3347 (2011).
Huttenhain, R. et al. Reproducible quantification of cancer-associated proteins in body fluids using targeted proteomics. Sci. Transl. Med. 4, 142ra94 (2012).
Dellaire, G., Berman, J. N. & Arceci, R. (Eds) Cancer genomics, from bench to personalized medicine (Elsevier, 2014).
Vegvari, A. et al. Identification of a novel proteoform of prostate specific antigen (SNP-L132I) in clinical samples by multiple reaction monitoring. Mol. Cell. Proteomics 12, 2761–2773 (2013).
Wu, C. et al. Integrated genome and transcriptome sequencing identifies a novel form of hybrid and aggressive prostate cancer. J. Pathol. 227, 53–61 (2012).
Saraon, P. et al. Quantitative proteomics reveals that enzymes of the ketogenic pathway are associated with prostate cancer progression. Mol. Cell. Proteomics 12, 1589–1601 (2013).
Drabovich, A. P., Pavlou, M. P., Dimitromanolakis, A. & Diamandis, E. P. Quantitative analysis of energy metabolic pathways in MCF-7 breast cancer cells by selected reaction monitoring assay. Mol. Cell. Proteomics 11, 422–434 (2012).
Olivetti, L. & Grazioli, L. (Eds) Imaging of urogenital diseases: a color atlas (Springer, 2009).
Lawrence, E. M., Gnanapragasam, V. J., Priest, A. N. & Sala, E. The emerging role of diffusion-weighted MRI in prostate cancer management. Nat. Rev. Urol. 9, 94–101 (2012).
Picchio, M. et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur. Urol. 59, 51–60 (2011).
Hirsh, A. Post-coital sperm retrieval could lead to the wider approval of assisted conception by some religions. Hum. Reprod. 11, 245–247 (1996).
Yamakawa, K., Yoshida, K., Nishikawa, H., Kato, T. & Iwamoto, T. Comparative analysis of interindividual variations in the seminal plasma proteome of fertile men with identification of potential markers for azoospermia in infertile patients. J. Androl. 28, 858–865 (2007).
Drake, R. R. et al. In-depth proteomic analyses of direct expressed prostatic secretions. J. Proteome Res. 9, 2109–2116 (2010).
Principe, S. et al. Identification of prostate-enriched proteins by in-depth proteomic analyses of expressed prostatic secretions in urine. J. Proteome Res. 11, 2386–2396 (2012).
Ponten, F. et al. A global view of protein expression in human cells, tissues, and organs. Mol. Syst. Biol. 5, 337 (2009).
Yang, L., Nyalwidhe, J. O., Guo, S., Drake, R. R. & Semmes, O. J. Targeted identification of metastasis-associated cell-surface sialoglycoproteins in prostate cancer. Mol. Cell. Proteomics 10, M110.007294 (2011).
Li, J. et al. Mapping of the human testicular proteome and its relationship with that of the epididymis and spermatozoa. Mol. Cell. Proteomics 10, M110.004630 (2011).
Li, J. et al. Systematic mapping and functional analysis of a family of human epididymal secretory sperm-located proteins. Mol. Cell. Proteomics 9, 2517–2528 (2010).
Johnston, D. S., Wooters, J., Kopf, G. S., Qiu, Y. & Roberts, K. P. Analysis of the human sperm proteome. Ann. NY Acad. Sci. 1061, 190–202 (2005).
Amaral, A. et al. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell. Proteomics 12, 330–342 (2013).
Baker, M. A. et al. Head and flagella subcompartmental proteomic analysis of human spermatozoa. Proteomics 13, 61–74 (2013).
Litwin, M. S. & Saigal, C. S. in Urologic diseases in America (eds Litwin, M. S. & Saigal, C. S.) 1–738 (US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007).
Feldman, D. R., Bosl, G. J., Sheinfeld, J. & Motzer, R. J. Medical treatment of advanced testicular cancer. JAMA 299, 672–684 (2008).
de Mouzon, J. et al. Assisted reproductive technology in Europe, 2007: results generated from European registers by ESHRE. Hum. Reprod. 27, 954–966 (2012).
Acknowledgements
We thank A. Dimitromanolakis and N. Musrap for help with the preparation of figures. The work of the authors was funded by grants from the Canadian Institutes of Health Research (CIHR) and Physician's Services Incorporated. A.P.D. was supported by CIHR and Mount Sinai Hospital Foundation Norm Hollend Post-Doctoral Fellowships.
Author information
Authors and Affiliations
Contributions
A.P.D. researched data for the article. A.P.D., K.J. and E.P.D. made substantial contributions to the discussion of content. A.P.D. and P.S. contributed to writing the article, and all authors contributed to review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.P.D., K.J. and E.P.D. have applied for a patent titled “Markers of the male urogenital tract,” international PCT application WO/2012/021969. P.S. declares no competing interests.
Rights and permissions
About this article
Cite this article
Drabovich, A., Saraon, P., Jarvi, K. et al. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat Rev Urol 11, 278–288 (2014). https://doi.org/10.1038/nrurol.2014.74
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2014.74
This article is cited by
-
Seminal plasma biomarkers for predicting successful sperm retrieval in patients with nonobstructive azoospermia: a narrative review of human studies
Basic and Clinical Andrology (2023)
-
Comparative proteomic analysis of seminal plasma exosomes in buffalo with high and low sperm motility
BMC Genomics (2023)
-
Low-polarity untargeted metabolomic profiling as a tool to gain insight into seminal fluid
Metabolomics (2023)
-
Overview of seminal fluid biomarkers for the evaluation of chronic prostatitis: a scoping review
Prostate Cancer and Prostatic Diseases (2022)
-
HIV-infection and cocaine use regulate semen extracellular vesicles proteome and miRNAome in a manner that mediates strategic monocyte haptotaxis governed by miR-128 network
Cellular and Molecular Life Sciences (2022)