Abstract
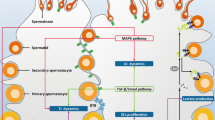
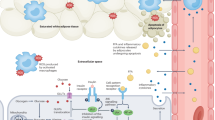
Male factor infertility is increasing in developed countries, and several factors linked to lifestyle have been shown to negatively affect spermatogenesis. Sertoli cells are pivotal to spermatogenesis, providing nutritional support to germ cells throughout their development. Sertoli cells display atypical features in their cellular metabolism; they can metabolize various substrates, preferentially glucose, the majority of which is converted to lactate and not oxidized via the tricarboxylic acid cycle. Why Sertoli cells preferentially export lactate for germ cells is not entirely understood. However, lactate is utilized as the main energy substrate by developing germ cells and has an antiapoptotic effect on these cells. Several biochemical mechanisms contribute to the modulation of lactate secretion by Sertoli cells. These include the transport of glucose through the plasma membrane, mediated by glucose transporters; the interconversion of pyruvate to lactate by lactate dehydrogenase; and the release of lactate mediated by monocarboxylate transporters. Several factors that modulate Sertoli cell metabolism have been identified, including sex steroid hormones, which are crucial for maintenance of energy homeostasis, influencing the metabolic balance of the whole body. In fact, energy status is essential for normal reproductive function, since the reproductive axis has the capacity to respond to metabolic cues.
Key Points
-
Sertoli cells have multiple roles in germ cell development, ranging from physical support and immunoprotection to the supply of nutrients and other factors
-
Germ cells have specific metabolic needs, which change during their development into spermatozoa, rendering them dependent on the nurturing provided by Sertoli cells
-
Sertoli cells utilize a number of different substrates (including glucose and fatty acids) and pathways to fulfill their metabolic requirements, as well as those of developing germ cells
-
A number of hormones and factors, such as follicle-stimulating hormone, insulin, insulin growth factor-I, epidermal growth factor, paracrine factor P-Mod-S, tri-iodothyronine, basic fibroblast growth factor, cytokines, carnitine, AMP-activated protein kinase, arachidonic acid and sex steroid hormones, are known to be metabolic modulators of Sertoli cells
-
Metabolic status is central to the regulation of the energy demands of the reproductive system, and extreme metabolic disorder conditions (such as obesity) are deleterious to reproductive function
-
The reproductive axis (hypothalamus–pituitary–testis axis) is exceptionally sensitive to energetic imbalance and disturbances of this axis severely affect Sertoli cells functions
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jorgensen, N. et al. Regional differences in semen quality in Europe. Hum. Reprod. 16, 1012–1019 (2001).
Jorgensen, N. et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum. Reprod. 17, 2199–2208 (2002).
Fernandez, M. et al. Semen quality and reproductive hormone levels in men from Southern Spain. Int. J. Androl. 35, 1–10 (2012).
Nordkap, L., Joensen, U. N., Jensen, M. B. & Jorgensen, N. Regional differences and temporal trends in male reproductive health disorders: Semen quality may be a sensitive marker of environmental exposures. Mol. Cell. Endocrinol. http://dx.doi.org/10.1016/j.mce.2011.05.048.
Bustos-Obregón, E. & Hartley, B. Ecotoxicology and testicular damage (environmental chemical pollution): a review. Int. J. Morphol. 26, 833–840 (2008).
Mathur, P. P. & D'Cruz, S. C. The effect of environmental contaminants on testicular function. Asian J. Androl. 13, 1–7 (2011).
Sharpe, R. M. Environmental/lifestyle effects on spermatogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 365, 1697–1712 (2010).
Goulis, D. G. & Tarlatzis, B. C. Metabolic syndrome and reproduction: I. testicular function. Gynecol. Endocrinol. 24, 33–39 (2008).
Mah, P. M. & Wittert, G. A. Obesity and testicular function. Mol. Cell. Endocrinol. 316, 180–186 (2010).
Bonde, J. P. & Storgaard, L. How work place conditions, environmental toxicants and lifestyle affect male reproductive function. Int. J. Androl. 25, 262–268 (2002).
Suehiro, R. M. et al. Testicular Sertoli cell function in male systemic lupus erythematosus. Rheumatology 47, 1692–1697 (2008).
Karagiannis, A. & Harsoulis, F. Gonadal dysfunction in systemic diseases. Eur. J. Endocrinol. 152, 501–513 (2005).
Sartorius, G. A. & Handelsman, D. J. in Andrology: Male Reproductive Health and Dysfunction (eds Nieschlag, E., Behre, H. M. & Nieschlag, S.) 339–364 (Springer, Berlin, 2010).
Su, L., Mruk, D. D. & Cheng, C. Y. Drug transporters, the blood-testis barrier and spermatogenesis. J. Endocrinol. 208, 207–223 (2011).
Setchell, B. P. The functional-significance of the blood-testis barrier. J. Androl. 1, 3–10 (1980).
Wong, C. H. & Cheng, C. Y. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr. Top. Dev. Biol. 71, 263–296 (2005).
Boussouar, F. & Benahmed, M. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. 15, 345–350 (2004).
Jutte, N., Grootegoed, J., Rommerts, F. & Van der Molen, H. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. Reproduction 62, 399 (1981).
Grootegoed, J., Oonk, R., Jansen, R. & Van der Molen, H. Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. Reproduction 77, 109 (1986).
Robinson, R. & Fritz, I. Metabolism of glucose by Sertoli cells in culture. Biol. Reprod. 24, 1032–1041 (1981).
Rato, L., Alves, M. G., Socorro, S., Cavaco, J. E. & Oliveira, P. F. in Endothelium and Epithelium: Composition, Functions and Pathology (eds Carrasco, J. & Matheus, M.) 137–155 (Nova Biomedical, New York, 2011).
Mruk, D. D. & Cheng, C. Y. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 (2004).
Toyama, Y., Maekawa, M. & Yuasa, S. Ectoplasmic specializations in the Sertoli cell: new vistas based on genetic defects and testicular toxicology. Anat. Sci. Int. 78, 1–16 (2003).
Mazaud-Guittot, S. et al. Claudin 11 deficiency in mice results in loss of the Sertoli cell epithelial phenotype in the testis. Biol. Reprod. 82, 202–213 (2010).
Lui, W. Y. & Cheng, C. Y. Regulation of cell junction dynamics by cytokines in the testis: a molecular and biochemical perspective. Cytokine Growth Factor Rev. 18, 299–311 (2007).
Cheng, C. Y. & Mruk, D. D. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit. Rev. Biochem. Mol. Biol. 44, 245–263 (2009).
Cheng, C. Y., Wong, E. W., Yan, H. H. & Mruk, D. D. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol. Cell. Endocrinol. 315, 49–56 (2010).
Waites, G. & Gladwell, R. Physiological significance of fluid secretion in the testis and blood-testis barrier. Phys. Rev. 62, 624–671 (1982).
Russell, L. D. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat. Rec. 190, 99–111 (1978).
Siu, M. K. Y. & Cheng, C. Y. in Molecular Mechanisms in Spermatogenesis (ed. Cheng, C. Y.) 74–91 (Landes Bioscience, Austin, 2009).
Setchell, B. P. The movement of fluids and substances in the testis. Aus. J. Biol. Sci. 39, 193–207 (1986).
Setchell, B. P. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv. Exp. Med. Biol. 636, 212–233 (2009).
Gaemers, I. C. et al. Differential expression pattern of retinoid X receptors in adult murine testicular cells implies varying roles for these receptors in spermatogenesis. Biol. Reprod. 58, 1351–1356 (1998).
Hogarth, C. A. & Griswold, M. D. The key role of vitamin A in spermatogenesis. J. Clin. Invest. 120, 956 (2010).
Sugimoto, R., Nabeshima, Y. & Yoshida, S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech. Dev. 128, 610–624 (2011).
Griswold, M. & McLean, D. in Knobil and Neill's Physiology of Reproduction (ed. Neill, J.) 949–975 (Elsevier, San Diego, 2006).
Dym, M. The fine structure of monkey Sertoli cells in the transitional zone at the junction of the seminiferous tubules with the tubuli recti. Am. J. Anat. 140, 1–25 (1974).
Russell, L., Ettlin, R., Sinha Hikim, A. & Clegg, E. Histological and Histopathological Evaluation of the Testis (Cache River Press, Clearwater, 1990).
Russell, L. D., Ren, H. P., Hikim, I. S., Schulze, W. & Hikim, A. P. S. A comparative study in twelve mammalian species of volume densities, volumes, and numerical densities of selected testis components, emphasizing those related to the Sertoli cell. Am. J. Anat. 188, 21–30 (1990).
Weber, J. E., Russell, L. D., Wong, V. & Peterson, R. N. Three-dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli--Sertoli and Sertoli--germ-cell relationships. Am. J. Anat. 167, 163–179 (1983).
O'Donnell, L., Robertson, K., Jones, M. & Simpson, E. Estrogen and spermatogenesis. Endocr. Rev. 22, 289–318 (2001).
Hess, R. & de Franca, L. in Molecular Mechanisms in Spermatogenesis (ed. Cheng, C. Y.) 1–15 (Landes Bioscience/Springer Science, Austin, 2008).
Rato, L., Socorro, S., Cavaco, J. & Oliveira, P. F. Tubular fluid secretion in the seminiferous epithelium: ion transporters and aquaporins in Sertoli cells. J. Memb. Biol. 236, 215–224 (2010).
Oliveira, P. F., Sousa, M., Barros, A., Moura, T. & Rebelo da Costa, A. Membrane transporters and cytoplasmatic pH regulation on bovine Sertoli cells. J. Memb. Biol. 227, 49–55 (2009).
Aly, H. A., Lightfoot, D. A. & El-Shemy, H. A. Bacterial lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Toxicol. In Vitro 24, 1266–1272 (2010).
Bajpai, M., Gupta, G. & Setty, B. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 138, 322–327 (1998).
Setchell, B. P. Hormones: what the testis really sees. Reprod. Fertil. Dev. 6, 535–545 (2004).
Wenger, R. H. & Katschinski, D. M. The hypoxic testis and post-meiotic expression of PAS domain proteins. Semin. Cell Dev. Biol. 16, 547–553 (2005).
Gómez, M. et al. Switches in 6-phosphofructo-2-kinase isoenzyme expression during rat sperm maturation. Biochem. Biophys. Res. Comm. 387, 330–335 (2009).
Courtens, J. L. & Ploen, L. Improvement of spermatogenesis in adult cryptorchid rat testis by intratesticular infusion of lactate. Biol. Reprod. 61, 154–161 (1999).
Erkkila, K., Aito, H., Aalto, K., Pentikainen, V. & Dunkel, L. Lactate inhibits germ cell apoptosis in the human testis. Mol. Hum. Reprod. 8, 109 (2002).
Nakamura, M., Fujiwara, A., Yasumasu, I., Okinaga, S. & Arai, K. Regulation of glucose metabolism by adenine nucleotides in round spermatids from rat testes. J. Biol. Chem. 257, 13945–13950 (1982).
Yanez, A. J. et al. Expression of key substrate cycle enzymes in rat spermatogenic cells: fructose 1, 6 bisphosphatase and 6 phosphofructose 1-kinase. J. Cell. Physiol. 212, 807–816 (2007).
Beckman, J. K. & Coniglio, J. G. A comparative study of the lipid composition of isolated rat Sertoli and germinal cells. Lipids 14, 262–267 (1979).
Lynch, K. M. Jr & Scott, W. W. Lipid distribution in the Sertoli cell and Leydig cell of the rat testis as related to experimental alterations of the pituitary-gonad system. Endocrinology 49, 8–14 (1951).
Retterstøl, K., Tran, T. N., Haugen, T. B. & Christophersen, B. O. Metabolism of very long chain polyunsaturated fatty acids in isolated rat germ cells. Lipids 36, 601–606 (2001).
Retterstol, K., Haugen, T. B., Tran, T. N. & Christophersen, B. O. Studies on the metabolism of essential fatty acids in isolated human testicular cells. Reproduction 121, 881–887 (2001).
Angulo, C. et al. Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J. Cell. Biochem. 71, 189–203 (1998).
Carosa, E. et al. Ontogenetic profile and thyroid hormone regulation of type-1 and type-8 glucose transporters in rat Sertoli cells. Int. J. Androl. 28, 99–106 (2005).
Galardo, M. et al. Regulation of expression of Sertoli cell glucose transporters 1 and 3 by FSH, IL1, and bFGF at two different time-points in pubertal development. Cell Tissue Res. 334, 295–304 (2008).
Ulisse, S., Jannini, E. A., Pepe, M., De Matteis, S. & D'Armiento, M. Thyroid hormone stimulates glucose transport and GLUT1 mRNA in rat Sertoli cells. Mol. Cell. Endocrinol. 87, 131–137 (1992).
Kokk, K. et al. Immunohistochemical detection of glucose transporters class I subfamily in the mouse, rat and human testis. Medicina (Kaunas) 40, 156–160 (2004).
Piroli, G. G. et al. Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J. Comp. Neurol. 452, 103–114 (2002).
Reagan, L. P. et al. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc. Natl Acad. Sci. USA 98, 2820–2825 (2001).
Oliveira, P. F. et al. Influence of 5alpha-dihydrotestosterone and 17beta-estradiol on human Sertoli cells metabolism. Int. J. Androl. 34, e612–e620 (2011).
Rato, L. et al. Metabolic modulation induced by estradiol and DHT in immature rat Sertoli cells cultured in vitro. Bioscience Reports 32, 61–69 (2012).
Riera, M. F., Galardo, M. N., Pellizzari, E. H., Meroni, S. B. & Cigorraga, S. B. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am. J. Physiol. Endocrinol. Metab. 297, 907–914 (2009).
Galardo, M. N., Riera, M. F., Pellizzari, E. H., Cigorraga, S. B. & Meroni, S. B. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-D-ribonucleoside, regulates lactate production in rat Sertoli cells. J. Mol. Endocrinol. 39, 279–288 (2007).
Tosca, L., Chabrolle, C. & Dupont, J. AMPK: a link between metabolism and reproduction? [French]. Med. Sci. 24, 297–300 (2008).
Zhang, B. B., Zhou, G. & Li, C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell. Metab. 9, 407–416 (2009).
Galardo, M. N. et al. Adenosine regulates Sertoli cell function by activating AMPK. Mol. Cell. Endocrinol. 330, 49–58 (2010).
Naimi, M., Arous, C. & Van Obberghen, E. Energetic cell sensors: a key to metabolic homeostasis. Trends Endocrinol. Metab. 21, 75–82 (2010).
Xiong, W. P., Wang, H. K., Wu, H., Chen, Y. M. & Han, D. S. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction 137, 469–479 (2009).
Leiderman, B. & Mancini, R. E. Glycogen content in the rat testis from postnatal to adult ages. Endocrinology 85, 607–609 (1969).
Slaughter, G. R. & Means, A. R. Follicle-stimulating hormone activation of glycogen phosphorylase in the Sertoli cell-enriched rat testis. Endocrinology 113, 1476–1485 (1983).
Lee, J., Richburg, J. H., Younkin, S. C. & Boekelheide, K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 138, 2081–2088 (1997).
Kaiser, G. R. et al. Metabolism of amino acids by cultured rat Sertoli cells. Metabolism: Clinical and Experimental 54, 515–521 (2005).
Oonk, R. B., Jansen, R. & Grootegoed, J. A. Differential effects of follicle-stimulating hormone, insulin, and insulin-like growth factor I on hexose uptake and lactate production by rat Sertoli cells. J. Cell. Physiol. 139, 210–218 (1989).
Oliveira, P. F. et al. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Bioch. Biophys. Acta 1820, 84–89 (2012).
Mallea, L. E., Machado, A. J., Navaroli, F. & Rommerts, F. F. Epidermal growth factor stimulates lactate production and inhibits aromatization in cultured Sertoli cells from immature rats. Int. J. Androl. 9, 201–208 (1986).
Mullaney, B. P., Rosselli, M. & Skinner, M. K. Developmental regulation of Sertoli cell lactate production by hormones and the testicular paracrine factor, PModS. Mol. Cell. Endocrinol. 104, 67–73 (1994).
Palmero, S., Prati, M., Bolla, F. & Fugassa, E. Tri-iodothyronine directly affects rat Sertoli cell proliferation and differentiation. J. Endocrinol. 145, 355–362 (1995).
Schteingart, H. F., Meroni, S. B., Canepa, D. F., Pellizzari, E. H. & Cigorraga, S. B. Effects of basic fibroblast growth factor and nerve growth factor on lactate production, gamma-glutamyl transpeptidase and aromatase activities in cultured Sertoli cells. Eur. J. Endocrinol. 141, 539–545 (1999).
Riera, M. F. et al. Regulation of lactate production by FSH, iL1beta, and TNFalpha in rat Sertoli cells. Gen. Comp. Endocrinol. 122, 88–97 (2001).
Meroni, S. B., Riera, M. F., Pellizzari, E. H., Schteingart, H. F. & Cigorraga, S. B. Possible role of arachidonic acid in the regulation of lactate production in rat Sertoli cells. Int. J. Androl. 26, 310–317 (2003).
Palmero, S., Bottazzi, C., Costa, M., Leone, M. & Fugassa, E. Metabolic effects of L-carnitine on prepubertal rat Sertoli cells. Horm. Metab. Res. 32, 87–90 (2000).
Guma, F. C., Wagner, M., Martini, L. H. & Bernard, E. A. Effect of FSH and insulin on lipogenesis in cultures of Sertoli cells from immature rats. Braz. J. Med. Biol. Res. 30, 591–597 (1997).
Riera, M., Meroni, S., Schteingart, H., Pellizzari, E. & Cigorraga, S. Regulation of lactate production and glucose transport as well as of glucose transporter 1 and lactate dehydrogenase A mRNA levels by basic fibroblast growth factor in rat Sertoli cells. J. Endocrinol. 173, 335–343 (2002).
Walker, W. H. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 365, 1557–1569 (2010).
Goddard, I. et al. Alteration of lactate production and transport in the adult rat testis exposed in utero to flutamide. Mol. Cell. Endocrinol. 206, 137–146 (2003).
Khan, U. W. & Rai, U. In vitro effect of FSH and testosterone on Sertoli cell nursing function in wall lizard Hemidactylus flaviviridis (Ruppell). Gen. Comp. Endocrinol. 136, 225–231 (2004).
Hurtado de Catalfo, G. E. & de Gomez Dumm, I. N. Influence of testosterone on polyunsaturated fatty acid biosynthesis in Sertoli cells in culture. Cell Biochem. Funct. 23, 175–180 (2005).
Fix, C., Jordan, C., Cano, P. & Walker, W. H. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc. Natl Acad. Sci. USA 101, 10919–10924 (2004).
Gupta, G., Srivastava, A. & Setty, B. Androgen-estrogen synergy in the regulation of energy metabolism in epididymis and vas deferens of rhesus monkey. Endocr. Res. 17, 383 (1991).
Denolet, E. et al. The effect of a Sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol. Endocrinol. 20, 321–334 (2006).
Crown, A., Clifton, D. K. & Steiner, R. A. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 86, 175–182 (2007).
Hill, J. W., Elmquist, J. K. & Elias, C. F. Hypothalamic pathways linking energy balance and reproduction. Am. J. Physiol. Endocrinol. Metab. 294, E827–E832 (2008).
Wade, G. N., Schneider, J. E. & Li, H. Y. Control of fertility by metabolic cues. Am. J. Physiol. 270, E1–E19 (1996).
Trumble, B. C., Brindle, E., Kupsik, M. & O'Connor, K. A. Responsiveness of the reproductive axis to a single missed evening meal in young adult males. Am. J. Hum. Biol. 22, 775–781 (2010).
Saradha, B. & Mathur, P. Effect of environmental contaminants on male reproduction. Environ. Toxicol. Pharmacol. 21, 34–41 (2006).
Nindl, B. C. et al. LH secretion and testosterone concentrations are blunted after resistance exercise in men. J. App. Physiol. 91, 1251–1258 (2001).
Chigrinskiy, E. & Conway, V. Protective effect of D-ribose against inhibition of rats testes function at excessive exercise. J. Stress Physiol. Biochem. 7, 242–249 (2011).
Petersen, C. & Soder, O. The Sertoli cell—a hormonal target and'super'nurse for germ cells that determines testicular size. Horm. Res. 66, 153–161 (2006).
Gonzalez, C. et al. Role of 17beta-estradiol and/or progesterone on insulin sensitivity in the rat: implications during pregnancy. J. Endocrinol. 166, 283 (2000).
Carreau, S. & Hess, R. A. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. B. Biol. Sci. 365, 1517–1535 (2010).
Smith, E. P. et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 331, 1056–1061 (1994).
Meyer, M. R., Clegg, D. J., Prossnitz, E. R. & Barton, M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol. 203, 259–269 (2011).
Pitteloud, N. et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabet. Care 28, 1636 (2005).
Alberti, K. G., Zimmet, P. & Shaw, J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 23, 469–480 (2006).
Cohen, P. G. Obesity in men: the hypogonadal-estrogen receptor relationship and its effect on glucose homeostasis. Med. Hypotheses 70, 358–360 (2008).
Hofstra, J. et al. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth. J. Med. 66, 103–109 (2008).
Moriarty-Kelsey, M., Harwood, J. E. F., Travers, S. H., Zeitler, P. S. & Nadeau, K. J. Testosterone, obesity and insulin resistance in young males: Evidence for an association between gonadal dysfunction and insulin resistance during puberty. J. Pediatr. Endocrinol. Metab. 23, 1281–1287 (2010).
Robeva, R., Tomova, A., Kirilov, G. & Kumanov, P. Anti-Mullerian hormone and inhibin B levels reflect altered Sertoli cell function in men with metabolic syndrome. Andrologia http://dx.doi.org/10.1111/j.1439-02722011.01185.x.
Martini, A. C. et al. Overweight and seminal quality: a study of 794 patients. Fertil. Steril. 94, 1739–1743 (2010).
Silva, F. R., Leite, L. D., Barreto, K. P., D'Agostini, C. & Zamoner, A. Effect of 3,5,3'-triiodo-L-thyronine on amino acid accumulation and membrane potential in Sertoli cells of the rat testis. Life Sci. 69, 977–986 (2001).
Mounzih, K., Lu, R. & Chehab, F. F. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 138, 1190–1193 (1997).
Steppan, C. M. et al. The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001).
Nogueiras, R. et al. Novel expression of resistin in rat testis: functional role and regulation by nutritional status and hormonal factors. J. Cell Sci. 117, 3247 (2004).
Rodriguez-Pacheco, F. et al. Regulation of pituitary cell function by adiponectin. Endocrinology 148, 401–410 (2007).
Caviglia, D., Scarabelli, L. & Palmero, S. Effects of carnitines on rat sertoli cell protein metabolism. Horm. Metab. Res. 36, 221–225 (2004).
Acknowledgements
This work was supported by the Portuguese “Fundação para a Ciência e a Tecnologia”—FCT (PTDC/QUI-BIQ/121446/2010) co-funded by FEDER via Programa Operacional Factores de Competitividade—COMPETE/QREN. L. Rato (SFRH/BD/72733/2010), M. G. Alves (SFRH/BPD/80451/2011) and A. I. Duarte (SFRH/BPD/26872/2006) were financed by FCT. P. F. Oliveira was financed by FCT through FSE and POPH funds (Programa Ciência 2008).
Author information
Authors and Affiliations
Contributions
L. Rato and M. G. Alves researched data for the article. L. Rato, M. G. Alves and P. F. Oliviera contributed substantially to discussion of content, writing and reviewing/editing the manuscript before submission. S. Socorro, A. I. Duarte and J. E. Cavaco contributed substantially to discussion of content and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rato, L., Alves, M., Socorro, S. et al. Metabolic regulation is important for spermatogenesis. Nat Rev Urol 9, 330–338 (2012). https://doi.org/10.1038/nrurol.2012.77
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2012.77
This article is cited by
-
Small noncoding RNAs and sperm nuclear basic proteins reflect the environmental impact on germ cells
Molecular Medicine (2024)
-
Metabolic regulation of proteome stability via N-terminal acetylation controls male germline stem cell differentiation and reproduction
Nature Communications (2023)
-
Morphology, Histology, and Transcriptome Analysis of Gonadal Development in Octopus minor (Sasaki, 1920)
Marine Biotechnology (2023)
-
STUB1 directs FOXQ1-mediated transactivation of Ldha gene and facilitates lactate production in mouse Sertoli cells
Cell and Tissue Research (2023)
-
Nicotinamide Mononucleotide Improves Spermatogenic Disorders in Aluminum-Exposed Rats by Modulating the Glycolytic Pathway
Biological Trace Element Research (2023)