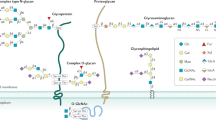
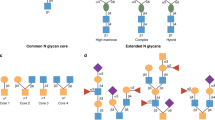
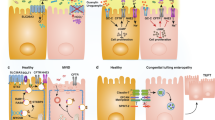
Key Points
-
Studies of the role of glycans in IBD are focused on three areas: glycosylation patterns of intestinal mucins, serum glycan levels of glycoproteins involved in inflammation, and colonic expression of glycan receptors
-
The carbohydrate content of mucus glycoproteins has been found to be reduced in patients with active ulcerative colitis compared with healthy controls
-
This finding provides an explanation for the role of glycans in IBD development, by suggesting that a defective inner mucus layer leads to increased bacterial contact with the epithelium that triggers inflammation
-
Decreased galactosylation of circulating IgG has been found in patients with ulcerative colitis compared with healthy controls, a finding that is relevant for effector functions of IgG
-
P-selectin and L-selectin are upregulated in IBD, which might be relevant for the development of new therapies, as pharmacological blockade of selectins has been demonstrated to ameliorate disease pathology in various diseases
-
The association between serum levels of mannose binding lectin and IBD has been analysed in many studies, but no link has been reported
Abstract
A number of genetic and immunological studies give impetus for investigating the role of glycosylation in IBD. Experimental mouse models have helped to delineate the role of glycosylation in intestinal mucins and to explore the putative pathogenic role of glycosylation in colitis. These experiments have been extended to human studies investigating the glycosylation patterns of intestinal mucins as well as levels of glycans of serum glycoproteins and expression of glycan receptors. These early human studies have generated interesting hypotheses regarding the pathogenic role of glycans in IBD, but have generally been restricted to fairly small underpowered studies. Decreased glycosylation has been observed in the intestinal mucus of patients with IBD, suggesting that a defective inner mucus layer might lead to increased bacterial contact with the epithelium, potentially triggering inflammation. In sera, decreased galactosylation of IgG has been suggested as a diagnostic marker for IBD. Advances in glycoprofiling technology make it technically feasible and affordable to perform high-throughput glycan pattern analyses and to build on previous work investigating a much wider range of glycan parameters in large numbers of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hart, G. W. & Copeland, R. J. Glycomics hits the big time. Cell 143, 672–676 (2010).
Zielinska, D. F., Gnad, F., Wisniewski, J. R. & Mann, M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141, 897–907 (2010).
Moran, A. P., Gupta, A. & Joshi, L. Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut 60, 1412–1425 (2011).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Murray, C. J. et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223 (2012).
Garrett, W. S., Gordon, J. I. & Glimcher, L. H. Homeostasis and inflammation in the intestine. Cell 140, 859–870 (2010).
van der Post, S. et al. Site-specific O-glycosylation on the MUC2 mucin protein inhibits cleavage by the Porphyromonas gingivalis secreted cysteine protease (RgpB). J. Biol. Chem. 288, 14636–14646 (2013).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Franke, A. et al. Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL). Nat. Genet. 42, 292–294 (2010).
Anderson, C. A. et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 43, 246–252 (2011).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Lauc, G. et al. Genomics meets glycomics-the first GWAS study of human N-glycome identifies HNF1α as a master regulator of plasma protein fucosylation. PLoS Genet. 6, e1001256 (2010).
Barrett, J. C. et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat. Genet. 41, 1330–1334 (2009).
McGovern, D. P. et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum. Mol. Genet. 19, 3468–3476 (2010).
Becker, D. J. & Lowe, J. B. Fucose: biosynthesis and biological function in mammals. Glycobiology 13, 41R–53R (2003).
Smith, P. L. et al. Conditional control of selectin ligand expression and global fucosylation events in mice with a targeted mutation at the FX locus. J. Cell Biol. 158, 801–815 (2002).
Lauc, G. et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 9, e1003225 (2013).
Kaneko, Y., Nimmerjahn, F. & Ravetch, J. V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006).
Thanabalasingham, G. et al. Mutations in HNF1A result in marked alterations of plasma glycan profile. Diabetes 62, 1329–1337 (2013).
Harvey, D. J., Merry, A. H., Royle, L., Campbell, M. P. & Rudd, P. M. Symbol nomenclature for representing glycan structures: Extension to cover different carbohydrate types. Proteomics 11, 4291–4295 (2011).
Moremen, K. W., Tiemeyer, M. & Nairn, A. V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 13, 448–462 (2012).
Gornik, O., Pavic, T. & Lauc, G. Alternative glycosylation modulates function of IgG and other proteins—implications on evolution and disease. Biochim. Biophys. Acta 1820, 1318–1326 (2012).
Zachara, N. E. & Hart, G. W. Cell signaling, the essential role of O-GlcNAc! Biochim. Biophys. Acta 1761, 599–617 (2006).
Marek, K. W., Vijay, I. K. & Marth, J. D. A recessive deletion in the GlcNAc-1-phosphotransferase gene results in peri-implantation embryonic lethality. Glycobiology 9, 1263–1271 (1999).
Freeze, H. H. Genetic defects in the human glycome. Nat. Rev. Genet. 7, 537–551 (2006).
Pucic, M. et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell Proteomics 10, M111.010090 (2011).
Knezevic, A. et al. Variability, heritability and environmental determinants of human plasma N-glycome. J. Proteome Res. 8, 694–701 (2009).
Pucic, M. et al. Common aberrations from the normal human plasma N-glycan profile. Glycobiology 20, 970–975 (2010).
National Research Council. Transforming Glycoscience: a Roadmap for the Future (the National Academies Press, 2012).
Johansson, M. E., Sjovall, H. & Hansson, G. C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 (2013).
Bennett, E. P. et al. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 (2012).
Holmen Larsson, J. M., Thomsson, K. A., Rodriguez-Pineiro, A. M., Karlsson, H. & Hansson, G. C. Studies of mucus in mouse stomach, small intestine, and colon. III. Gastrointestinal Muc5ac and Muc2 mucin O-glycan patterns reveal a regiospecific distribution. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G357–G363 (2013).
Ermund, A., Schutte, A., Johansson, M. E., Gustafsson, J. K. & Hansson, G. C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G341–G347 (2013).
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Fu, J. et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest. 121, 1657–1666 (2011).
Johansson, M. E. et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 (2014).
Johansson, M. E. et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 5, e12238 (2010).
Campbell, B. J., Finnie, I. A., Hounsell, E. F. & Rhodes, J. M. Direct demonstration of increased expression of Thomsen-Friedenreich (TF) antigen in colonic adenocarcinoma and ulcerative colitis mucin and its concealment in normal mucin. J. Clin. Invest. 95, 571–576 (1995).
Clamp, J. R., Fraser, G. & Read, A. E. Study of the carbohydrate content of mucus glycoproteins from normal and diseased colons. Clin. Sci. 61, 229–234 (1981).
Larsson, J. M. H. et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 17, 2299–2307 (2011).
Teague, R. H., Fraser, D. & Clamp, J. R. Changes in monosaccharide content of mucous glycoproteins in ulcerative colitis. BMJ 2, 645–646 (1973).
Cassel, S. L., Sutterwala, F. S. & Flavell, R. A. The tiny conductor: immune regulation via commensal organisms. Cell Host Microbe 3, 340–341 (2008).
Rhodes, J. M., Black, R. R. & Savage, A. Altered lectin binding by colonic epithelial glycoconjugates in ulcerative colitis and Crohn's disease. Dig. Dis. Sci. 33, 1359–1363 (1988).
Cooper, H. S., Farano, P. & Coapman, R. A. Peanut lectin binding sites in colons of patients with ulcerative colitis. Arch. Pathol. Lab. Med. 111, 270–275 (1987).
Boland, C. R. Mucin glycoproteins in chronic ulcerative colitis. Peanut lectin binding in human and nonhuman primate colons. Dig. Dis. Sci. 30, 147S–153S (1985).
Boland, C. R., Lance, P., Levin, B., Riddell, R. H. & Kim, Y. S. Abnormal goblet cell glycoconjugates in rectal biopsies associated with an increased risk of neoplasia in patients with ulcerative colitis: early results of a prospective study. Gut 25, 1364–1371 (1984).
Carneiro, F. et al. T (Thomsen-Friedenreich) antigen and other simple mucin-type carbohydrate antigens in precursor lesions of gastric carcinoma. Histopathology 24, 105–113 (1994).
Dabelsteen, E., Clausen, H., Holmstrup, P. & Reibel, J. Premalignant and malignant oral lesions are associated with changes in the glycosylation pattern of carbohydrates related to ABH blood group antigens. APMIS 96, 813–819 (1988).
Springer, G. F., Desai, P. R., Ghazizadeh, M. & Tegtmeyer, H. T/Tn pancarcinoma autoantigens: fundamental, diagnostic, and prognostic aspects. Cancer Detect. Prev. 19, 173–182 (1995).
Springer, G. F. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. (Berl.) 75, 594–602 (1997).
Springer, G. F. T and Tn, general carcinoma autoantigens. Science 224, 1198–1206 (1984).
Bodger, K. et al. Altered colonic glycoprotein expression in unaffected monozygotic twins of inflammatory bowel disease patients. Gut 55, 973–977 (2006).
Swidsinski, A. et al. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56, 343–350 (2007).
Johansson, M. E. et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 (2014).
Lidell, M. E., Moncada, D. M., Chadee, K. & Hansson, G. C. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc. Natl Acad. Sci. USA 103, 9298–9303 (2006).
An, G. et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 204, 1417–1429 (2007).
Camacho, F. I. et al. CD44v6 expression in inflammatory bowel disease is associated with activity detected by endoscopy and pathological features. Histopathology 35, 144–149 (1999).
Rosenberg, W. M. et al. Increased expression of CD44v6 and CD44v3 in ulcerative colitis but not colonic Crohn's disease. Lancet 345, 1205–1209 (1995).
Campbell, B. J., Yu, L. G. & Rhodes, J. M. Altered glycosylation in inflammatory bowel disease: a possible role in cancer development. Glycoconj. J. 18, 851–858 (2001).
Rottger, S. et al. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J. Cell Sci. 111, 45–60 (1998).
Campbell, B. J., Rowe, G. E., Leiper, K. & Rhodes, J. M. Increasing the intra-Golgi pH of cultured LS174T goblet-differentiated cells mimics the decreased mucin sulfation and increased Thomsen-Friedenreich antigen (Gal beta1–3GalNac alpha-) expression seen in colon cancer. Glycobiology 11, 385–393 (2001).
Kaneko, Y. et al. Altered expression of CDX-2, PDX-1 and mucin core proteins in “Ulcer-associated cell lineage (UACL)” in Crohn's disease. J. Mol. Histol. 39, 161–168 (2008).
Arnold, J. N., Saldova, R., Hamid, U. M. & Rudd, P. M. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics 8, 3284–3293 (2008).
Miyahara, K. et al. Serum glycan markers for evaluation of disease activity and prediction of clinical course in patients with ulcerative colitis. PLoS ONE 8, e74861 (2013).
Parekh, R. B. et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316, 452–457 (1985).
Davidson, A. & Diamond, B. Autoimmune diseases. N. Engl. J. Med. 345, 340–350 (2001).
Herszenyi, L. & Tulassay, Z. The role of autoantibodies in inflammatory bowel disease. Dig. Dis. 30, 201–207 (2012).
Surolia, I. et al. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature 466, 243–247 (2010).
Aschermann, S., Lux, A., Baerenwaldt, A., Biburger, M. & Nimmerjahn, F. The other side of immunoglobulin G: suppressor of inflammation. Clin. Exp. Immunol. 160, 161–167 (2010).
Fujii, S., Nishiura, T., Nishikawa, A., Miura, R. & Taniguchi, N. Structural heterogeneity of sugar chains in immunoglobulin, G. Conformation of immunoglobulin G molecule and substrate specificities of glycosyltransferases. J. Biol. Chem. 265, 6009–6018 (1990).
Karsten, C. M. et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat. Med. 18, 1401–1406 (2012).
Dube, R. et al. Agalactosyl IgG in inflammatory bowel disease: correlation with C-reactive protein. Gut 31, 431–434 (1990).
Shinzaki, S. et al. IgG oligosaccharide alterations are a novel diagnostic marker for disease activity and the clinical course of inflammatory bowel disease. Am. J. Gastroenterol. 103, 1173–1181 (2008).
Singh, K., Chang, C. & Gershwin, M. E. IgA deficiency and autoimmunity. Autoimmun. Rev. 13, 163–177 (2014).
Mattu, T. S. et al. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcalpha receptor interactions. J. Biol. Chem. 273, 2260–2272 (1998).
Inoue, T. et al. Deficiency of N-acetylgalactosamine in O-linked oligosaccharides of IgA is a novel biologic marker for Crohn's disease. Inflamm. Bowel Dis. 18, 1723–1734 (2012).
Inoue, T. et al. O-linked oligosaccharide alterations of IgA1 are a novel biological marker of patients with inflammatory bowel disease. Dig. Dis. Sci. 56, 2772 (2011).
Levy, A. P. et al. Haptoglobin: basic and clinical aspects. Antioxid. Redox Signal. 12, 293–304 (2010).
Treuheit, M. J., Costello, C. E. & Halsall, H. B. Analysis of the five glycosylation sites of human alpha 1-acid glycoprotein. Biochem. J. 283, 105–112 (1992).
Goodarzi, M. T. & Turner, G. A. Reproducible and sensitive determination of charged oligosaccharides from haptoglobin by PNGase F digestion and HPAEC/PAD analysis: glycan composition varies with disease. Glycoconj. J. 15, 469–475 (1998).
Park, S.-Y. et al. Dimeric Lea (Lea-on-Lea) status of β-haptoglobin in sera of colon cancer, chronic inflammatory disease and normal subjects. Int. J. Oncol. 36, 1291–1297 (2010).
Park, S. Y. et al. N-glycosylation status of β-haptoglobin in sera of patients with colon cancer, chronic inflammatory diseases and normal subjects. Int. J. Cancer 126, 142–155 (2010).
Park, S. Y. et al. α1–3/4 fucosylation at Asn 241 of β-haptoglobin is a novel marker for colon cancer: A combinatorial approach for development of glycan biomarkers. Int. J. Cancer 130, 2366–2376 (2012).
Ryden, I., Skude, G., Lundblad, A. & Pahlsson, P. Glycosylation of alpha1-acid glycoprotein in inflammatory disease: analysis by high-pH anion-exchange chromatography and concanavalin A crossed affinity immunoelectrophoresis. Glycoconj. J. 14, 481–488 (1997).
Ghazarian, H., Idoni, B. & Oppenheimer, S. B. A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 113, 236–247 (2011).
Smith, D. F., Song, X. & Cummings, R. D. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 480, 417–444 (2010).
Sharon, N. & Lis, H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14, 53R–62R (2004).
Wagner, D. D. & Frenette, P. S. The vessel wall and its interactions. Blood 111, 5271–5281 (2008).
Polinska, B., Matowicka-Karna, J. & Kemona, H. Assessment of the influence of the inflammatory process on the activation of blood platelets and morphological parameters in patients with ulcerative colitis (colitis ulcerosa). Folia Histochem. Cytobiol. 49, 119–124 (2011).
Pamuk, G. E. et al. Increased circulating platelet-neutrophil, platelet-monocyte complexes, and platelet activation in patients with ulcerative colitis: a comparative study. Am. J. Hematol. 81, 753–759 (2006).
Kayo, S. et al. Close association between activated platelets and neutrophils in the active phase of ulcerative colitis in humans. Inflamm. Bowel Dis. 12, 727–735 (2006).
Andoh, A. et al. Elevated circulating platelet-derived microparticles in patients with active inflammatory bowel disease. Am. J. Gastroenterol. 100, 2042–2048 (2005).
Irving, P. M. et al. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm. Bowel Dis. 10, 361–372 (2004).
Magro, F. et al. Soluble selectins, sICAM, sVCAM, and angiogenic proteins in different activity groups of patients with inflammatory bowel disease. Dig. Dis. Sci. 49, 1265–1274 (2004).
Suzuki, K. et al. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand. J. Gastroenterol. 36, 1301–1306 (2001).
Goke, M., Hoffmann, J. C., Evers, J., Kruger, H. & Manns, M. P. Elevated serum concentrations of soluble selectin and immunoglobulin type adhesion molecules in patients with inflammatory bowel disease. J. Gastroenterol. 32, 480–486 (1997).
Schurmann, G. M. et al. Increased expression of cell adhesion molecule P-selectin in active inflammatory bowel disease. Gut 36, 411–418 (1995).
Collins, C. E., Cahill, M. R., Newland, A. C. & Rampton, D. S. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology 106, 840–845 (1994).
Nakamura, S. et al. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Lab. Invest. 69, 77–85 (1993).
Huang, Q., Wang, E., Lin, M., Yan, X. & Zhang, Y. Analysis of P-selectin and platelet parameters in patients with ulcerative colitis. [Chinese]. Chin. J. Gastroenterol. 17, 430–432 (2012).
Gao, Y. H. et al. Relationship and significance between anti-beta2-glycoprotein I antibodies and platelet activation state in patients with ulcerative colitis. World J. Gastroenterol. 14, 771–775 (2008).
Zarbock, A., Polanowska-Grabowska, R. K. & Ley, K. Platelet–neutrophil–interactions: linking hemostasis and inflammation. Blood Rev. 21, 99–111 (2007).
Bedard, P. W. & Kaila, N. Selectin inhibitors: a patent review. Expert Opin. Ther. Pat. 20, 781–793 (2010).
Goggins, M. G. et al. Soluble adhesion molecules in inflammatory bowel disease. Irish J. Med. Sci. 170, 107–111 (2001).
Bhatti, M., Chapman, P., Peters, M., Haskard, D. & Hodgson, H. J. Visualising E-selectin in the detection and evaluation of inflammatory bowel disease. Gut 43, 40–47 (1998).
Cellier, C. et al. In-situ endothelial cell adhesion molecule expression in ulcerative colitis. E-selectin in-situ expression correlates with clinical, endoscopic and histological activity and outcome. Eur. J. Gastroenterol. Hepatol. 9, 1197–1203 (1997).
Nielsen, O. H., Brynskov, J. & Vainer, B. Increased mucosal concentrations of soluble intercellular adhesion molecule-1 (sICAM-1), sE-selectin, and interleukin-8 in active ulcerative colitis. Dig. Dis. Sci. 41, 1780–1785 (1996).
Patel, R. T., Pall, A. A., Adu, D. & Keighley, M. R. Circulating soluble adhesion molecules in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 7, 1037–1041 (1995).
Oshitani, N. et al. Adhesion molecule expression on vascular endothelium and nitroblue tetrazolium reducing activity in human colonic mucosa. Scand. J. Gastroenterol. 30, 915–920 (1995).
Pooley, N., Ghosh, L. & Sharon, P. Up-regulation of E-selectin and intercellular adhesion molecule-1 differs between Crohn's disease and ulcerative colitis. Dig. Dis. Sci. 40, 219–225 (1995).
Gulubova, M. V., Manolova, I. M., Vlaykova, T. I., Prodanova, M. & Jovchev, J. P. Adhesion molecules in chronic ulcerative colitis. Int. J. Colorect. Dis. 22, 581–589 (2007).
Arihiro, S. et al. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn's disease. Pathol. Int. 52, 367–374 (2002).
Lazaris, A. C., Dicoglou, C., Tseleni-Balafouta, S., Paraskevakou, H. & Davaris, P. S. In situ expression of E-selectin and intercellular adhesion molecule-1 in chronic inflammatory diseases of the gastrointestinal tract. APMIS 107, 819–827 (1999).
Vainer, B., Nielsen, O. H. & Horn, T. Expression of E-selectin, sialyl Lewis X, and macrophage inflammatory protein-1alpha by colonic epithelial cells in ulcerative colitis. Dig. Dis. Sci. 43, 596–608 (1998).
Jones, S. C. et al. Adhesion molecules in inflammatory bowel disease. Gut 36, 724–730 (1995).
Vainer, B. & Nielsen, O. H. Serum concentration and chemotactic activity of E-selectin (CD62E) in inflammatory bowel disease. Mediators Inflamm. 3, 215–218 (1994).
Rafiee, P. et al. Thalidomide inhibits inflammatory and angiogenic activation of human intestinal microvascular endothelial cells (HIMEC). Am. J. Physiol. Gastrointest. Liver Physiol. 298, G167–G176 (2010).
Ley, K. Sulfated sugars for rolling lymphocytes. J. Exp. Med. 198, 1285–1288 (2003).
Seidelin, J. B., Vainer, B., Horn, T. & Nielsen, O. H. Circulating L-selectin levels and endothelial CD34 expression in inflammatory bowel disease. Am. J. Gastroenterol. 93, 1854–1859 (1998).
Irving, P. M. et al. Platelet-leucocyte aggregates form in the mesenteric vasculature in patients with ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 20, 283–289 (2008).
Suzawa, K. et al. Preferential induction of peripheral lymph node addressin on high endothelial venule-like vessels in the active phase of ulcerative colitis. Am. J. Gastroenterol. 102, 1499–1509 (2007).
Kobayashi, M. et al. GlcNAc6ST-1-mediated decoration of MAdCAM-1 protein with L-selectin ligand carbohydrates directs disease activity of ulcerative colitis. Inflamm. Bowel Dis. 15, 697–706 (2009).
Feagan, B. G. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369, 699–710 (2013).
Sandborn, W. J. et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 369, 711–721 (2013).
Targan, S. R. et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology 132, 1672–1683 (2007).
Rivera-Nieves, J. et al. L-selectin, alpha 4 beta 1, and alpha 4 beta 7 integrins participate in CD4+ T cell recruitment to chronically inflamed small intestine. J. Immunol. 174, 2343–2352 (2005).
Schon, M. P., Drewniok, C. & Boehncke, W. H. Targeting selectin functions in the therapy of psoriasis. Curr. Drug Targets Inflamm. Allergy 3, 163–168 (2004).
Chataway, J. & Miller, D. H. Natalizumab therapy for multiple sclerosis. Neurotherapeutics 10, 19–28 (2013).
Danese, S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 61, 918–932 (2012).
Papp, M. et al. Mannose-binding lectin level and deficiency is not associated with inflammatory bowel diseases, disease phenotype, serology profile, and NOD2/CARD15 genotype in a large Hungarian cohort. Hum. Immunol. 71, 407–413 (2010).
Zimmermann-Nielsen, E., Baatrup, G., Thorlacius-Ussing, O., Agnholt, J. & Svehag, S. E. Complement activation mediated by mannan-binding lectin in plasma from healthy individuals and from patients with SLE, Crohn's disease and colorectal cancer. Suppressed activation by SLE plasma. Scand. J. Immunol. 55, 105–110 (2002).
Nakajima, S. et al. Functional analysis of agalactosyl IgG in inflammatory bowel disease patients. Inflamm. Bowel Dis. 17, 927–936 (2011).
Hoffmann, C. et al. Is there a role for mannan-binding lectin in the diagnosis of inflammatory bowel disease? Immunogenetics 62, 231–235 (2010).
Schoepfer, A. M. et al. Low Mannan-binding lectin serum levels are associated with complicated Crohn's disease and reactivity to oligomannan (ASCA). Am. J. Gastroenterol. 104, 2508–2516 (2009).
Seibold, F. et al. Association of deficiency for mannan-binding lectin with anti-mannan antibodies in Crohn's disease: a family study. Inflamm. Bowel Dis. 13, 1077–1082 (2007).
Nielsen, R. G. et al. Genetic polymorphisms of mannan binding lectin (MBL), serum levels of MBL, the MBL associated serine protease and H-ficolin in patients with Crohn's disease. Gut 56, 311–312 (2007).
Seibold, F. et al. Genetic variants of the mannan-binding lectin are associated with immune reactivity to mannans in Crohn's disease. Gastroenterology 127, 1076–1084 (2004).
Lippert, E. et al. Regulation of galectin-3 function in mucosal fibroblasts: potential role in mucosal inflammation. Clin. Exp. Immunol. 152, 285–297 (2008).
Shan, M. et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013).
Zhao, X. et al. Evaluation of p38 MAPK pathway as a molecular signature in ulcerative colitis. J. Proteome Res. 10, 2216–2225 (2011).
Muller, S. et al. Galectin-3 modulates T cell activity and is reduced in the inflamed intestinal epithelium in IBD. Inflamm. Bowel Dis. 12, 588–597 (2006).
Jensen-Jarolim, E. et al. The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of Crohn's disease patients, and tumour necrosis factor alpha decreases the level of galectin-3-specific mRNA in HCT-8 cells. Eur. J. Gastroenterol. Hepatol. 14, 145–152 (2002).
Frol'ová, L. et al. Detection of galectin-3 in patients with inflammatory bowel diseases: New serum marker of active forms of IBD? Inflamm. Res. 58, 503–512 (2009).
Morimoto, K. et al. Dysregulated upregulation of T-cell immunoglobulin and mucin domain-3 on mucosal T helper 1 cells in patients with Crohn's disease. Scand. J. Gastroenterol. 46, 701–709 (2011).
Shi, F. et al. Dysregulated Tim-3 expression and its correlation with imbalanced CD4 helper T cell function in ulcerative colitis. Clin. Immunol. 145, 230–240 (2012).
Masuda, K. et al. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol. Immunol. 44, 3122–3131 (2007).
Iida, S. et al. Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcγRIIIa. Clin. Cancer Res. 12, 2879–2887 (2006).
Scanlan, C. N., Burton, D. R. & Dwek, R. A. Making autoantibodies safe. Proc. Natl Acad. Sci. USA 105, 4081–4082 (2008).
Preithner, S. et al. High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immunoglobulin G. Mol. Immunol. 43, 1183–1193 (2006).
Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003).
Kolarich, D. et al. The minimum information required for a glycomics experiment (MIRAGE) project: improving the standards for reporting mass-spectrometry-based glycoanalytic data. Mol. Cell. Proteomics 12, 991–995 (2013).
Landis, S. C. et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490, 187–191 (2012).
Gornik, O. & Lauc, G. Glycosylation of serum proteins in inflammatory diseases. Dis. Markers 25, 267–278 (2008).
Gornik, O. & Lauc, G. Enzyme linked lectin assay (ELLA) for direct analysis of transferrin sialylation in serum samples. Clin. Biochem. 40, 718–723 (2007).
Stockmann, H., Adamczyk, B., Hayes, J. & Rudd, P. M. Automated, high-throughput IgG-antibody glycoprofiling platform. Anal. Chem. 85, 8841–8849 (2013).
Austrup, F. et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature 385, 81–83 (1997).
Lasky, L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science 258, 964–969 (1992).
Lasky, L. A. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu. Rev. Biochem. 64, 113–139 (1995).
St Hill, C. A. Interactions between endothelial selectins and cancer cells regulate metastasis. Front. Biosci. (Landmark Ed.) 16, 3233–3251 (2011).
Genbacev, O. D. et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 299, 405–408 (2003).
Mitoma, J. et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat. Immunol. 8, 409–418 (2007).
Homeister, J. W. et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 15, 115–126 (2001).
Nimrichter, L. et al. Intact cell adhesion to glycan microarrays. Glycobiology 14, 197–203 (2004).
Rabinovich, G. A., Liu, F. T., Hirashima, M. & Anderson, A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 66, 143–158 (2007).
Rabinovich, G. A. & Toscano, M. A. Turning 'sweet' on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 (2009).
Acknowledgements
E.T. and H.C. are joint first authors, and J.S. and G.L. are joint last authors. The authors acknowledge the work of the IBD-BIOM group.
Author information
Authors and Affiliations
Contributions
E.T. researched data, and contributed to discussion of content and writing the article. H.C., D.K., M.W., N.A.K., E.N. and J.S. contributed to the discussion of content and reviewing/editing the manuscript before submission. N.V., D.P.B.M. and G.L. contributed to writing the article and reviewing/editing it before submission. M.P.B., V.A., D.F., I.K.P., I.R. and V.Z. reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.P.B. works for Genos Ltd, a private research organization that specializes in high-throughput glycomic analysis. D.F. is the CEO of Ludger Ltd, a commercial company that specializes in the development and validation of glycoprofiling technology for biologic therapeutics and biological tissues. There are no patents, products in development or marketed products to declare. I.K.P. is the Research Director of IP Research Consulting SAS, a privately-owned, research intensive SME under the commercial name of Photeomix Protein Discovery that specializes in the discovery and validation of biomarkers based on post-translational protein modification activities. There are no patents, products in development or marketed products to declare. G. L. is founder and owner of Genos Ltd. These competing interests do not alter the authors' adherence to all the Nature Publishing Group rules or other rules that might be perceived to influence the interpretation of the article. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Theodoratou, E., Campbell, H., Ventham, N. et al. The role of glycosylation in IBD. Nat Rev Gastroenterol Hepatol 11, 588–600 (2014). https://doi.org/10.1038/nrgastro.2014.78
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.78
This article is cited by
-
Causal relationship between gut Prevotellaceae and risk of sepsis: a two-sample Mendelian randomization and clinical retrospective study in the framework of predictive, preventive, and personalized medicine
EPMA Journal (2023)
-
Trans-Golgi protein TVP23B regulates host-microbe interactions via Paneth cell homeostasis and Goblet cell glycosylation
Nature Communications (2023)
-
Unraveling function and diversity of bacterial lectins in the human microbiome
Nature Communications (2022)
-
Finding the sweet spot: glycosylation mediated regulation of intestinal inflammation
Mucosal Immunology (2022)
-
Diversity and dynamism of IgA−microbiota interactions
Nature Reviews Immunology (2021)