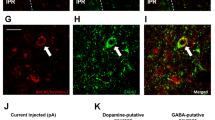
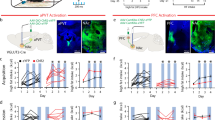
Abstract
The prevalence of obesity has markedly increased over the past few decades. Exploration of how hunger and satiety signals influence the reward system can help us understand non-homeostatic feeding. Insulin may act in the ventral tegmental area (VTA), a critical site for reward-seeking behavior, to suppress feeding. However, the neural mechanisms underlying insulin effects in the VTA remain unknown. We demonstrate that insulin, a circulating catabolic peptide that inhibits feeding, can induce long-term depression (LTD) of mouse excitatory synapses onto VTA dopamine neurons. This effect requires endocannabinoid-mediated presynaptic inhibition of glutamate release. Furthermore, after a sweetened high-fat meal, which elevates endogenous insulin, insulin-induced LTD is occluded. Finally, insulin in the VTA reduces food anticipatory behavior in mice and conditioned place preference for food in rats. Taken together, these results suggest that insulin in the VTA suppresses excitatory synaptic transmission and reduces anticipatory activity and preference for food-related cues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
04 February 2013
In the version of this article initially published online, the y axis of Figure 1f was labeled AMPA EPSCs; the graph actually shows GABAA IPSCs. The error has been corrected for the print, PDF and HTML versions of this article.
References
Gerozissis, K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur. J. Pharmacol. 585, 38–49 (2008).
Palmiter, R.D. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 30, 375–381 (2007).
Bonci, A. & Malenka, R.C. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J. Neurosci. 19, 3723–3730 (1999).
Overton, P.G., Richards, C.D., Berry, M.S. & Clark, D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. Neuroreport 10, 221–226 (1999).
Borgland, S.L. et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J. Neurosci. 29, 11215–11225 (2009).
Borgland, S.L., Taha, S.A., Sarti, F., Fields, H.L. & Bonci, A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49, 589–601 (2006).
Abizaid, A. et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Invest. 116, 3229–3239 (2006).
Thompson, J.L. & Borgland, S.L. A role for hypocretin/orexin in motivation. Behav. Brain Res. 217, 446–453 (2011).
Baskin, D.G. et al. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 848, 114–123 (1999).
Woods, S.C., Seeley, R.J., Baskin, D.G. & Schwartz, M.W. Insulin and the blood-brain barrier. Curr. Pharm. Des. 9, 795–800 (2003).
Hommel, J.D. et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810 (2006).
Fulton, S. et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51, 811–822 (2006).
Figlewicz, D.P., Evans, S.B., Murphy, J., Hoen, M. & Baskin, D.G. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 964, 107–115 (2003).
Figlewicz, D.P., Bennett, J.L., Naleid, A.M., Davis, C. & Grimm, J.W. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol. Behav. 89, 611–616 (2006).
Figlewicz, D.P. et al. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav. Neurosci. 118, 479–487 (2004).
Sipols, A.J., Bayer, J., Bennett, R. & Figlewicz, D.P. Intraventricular insulin decreases kappa opioid-mediated sucrose intake in rats. Peptides 23, 2181–2187 (2002).
Figlewicz, D.P., Bennett, J.L., Aliakbari, S., Zavosh, A. & Sipols, A.J. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R388–R394 (2008).
Mebel, D.M., Wong, J.C., Dong, Y.J. & Borgland, S.L. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur. J. Neurosci. 36, 2336–2346 (2012).
Bruijnzeel, A.W., Corrie, L.W., Rogers, J.A. & Yamada, H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav. Brain Res. 219, 254–264 (2011).
Overton, P.G. & Clark, D. Burst firing in midbrain dopamine neurons. Brain Res. Brain Res. Rev. 25, 312–334 (1997).
Watabe-Uchida, M., Zhu, L., Ogawa, S.K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Wang, Y.T. & Linden, D.J. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 25, 635–647 (2000).
Man, H.Y. et al. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 25, 649–662 (2000).
Baltensperger, K. et al. Catalysis of serine and tyrosine autophosphorylation by the human insulin receptor. Proc. Natl. Acad. Sci. USA 89, 7885–7889 (1992).
Schäffer, L. et al. A novel high-affinity peptide antagonist to the insulin receptor. Biochem. Biophys. Res. Commun. 376, 380–383 (2008).
Vigneri, R., Squatrito, S. & Sciacca, L. Insulin and its analogs: actions via insulin and IGF receptors. Acta Diabetol. 47, 271–278 (2010).
Asnaghi, L., Bruno, P., Priulla, M. & Nicolin, A. mTOR: a protein kinase switching between life and death. Pharmacol. Res. 50, 545–549 (2004).
Mameli, M., Balland, B., Lujan, R. & Lüscher, C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317, 530–533 (2007).
Lee, S.H., Liu, L., Wang, Y.T. & Sheng, M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36, 661–674 (2002).
Malinow, R. & Malenka, R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002).
Lüscher, C. et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 24, 649–658 (1999).
Kauer, J.A. & Malenka, R.C. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 8, 844–858 (2007).
Katz, B. Quantal mechanism of neural transmitter release. Science 173, 123–126 (1971).
Melis, M. et al. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J. Neurosci. 24, 53–62 (2004).
Heifets, B.D. & Castillo, P.E. Endocannabinoid signaling and long-term synaptic plasticity. Annu. Rev. Physiol. 71, 283–306 (2009).
Bisogno, T. et al. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem. Biophys. Res. Commun. 256, 377–380 (1999).
Blum, I.D., Waddington-Lamont, E., Rodrigues, T. & Abizaid, A. Isolating neural correlates of the pacemaker for food anticipation. PLoS ONE 7, e36117 (2012).
Ahn, S. & Phillips, A.G. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. J. Neurosci. 19, RC29 (1999).
Sanchis-Segura, C. & Spanagel, R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 11, 2–38 (2006).
Pan, B., Hillard, C.J. & Liu, Q.S. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J. Neurosci. 28, 1385–1397 (2008).
Henny, P. et al. Structural correlates of heterogeneous in vivo activity of midbrain dopaminergic neurons. Nat. Neurosci. 15, 613–619 (2012).
Grueter, B.A., Brasnjo, G. & Malenka, R.C. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci. 13, 1519–1525 (2010).
Gerdeman, G.L., Ronesi, J. & Lovinger, D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 5, 446–451 (2002).
Ronesi, J., Gerdeman, G.L. & Lovinger, D.M. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J. Neurosci. 24, 1673–1679 (2004).
Yin, H.H., Davis, M.I., Ronesi, J.A. & Lovinger, D.M. The role of protein synthesis in striatal long-term depression. J. Neurosci. 26, 11811–11820 (2006).
Massa, F. et al. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J. Neurosci. 30, 6273–6281 (2010).
Lüscher, C. & Malenka, R.C. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69, 650–663 (2011).
Duckworth, W.C., Bennett, R.G. & Hammel, F.G. Insulin degradation: progress and potential. Endocr. Rev. 19, 608–624 (1998).
Tsai, H.C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Stuber, G.D. et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 321, 1690–1692 (2008).
Yoshizaki, T. et al. Isolation and transplantation of dopaminergic neurons generated from mouse embryonic stem cells. Neurosci. Lett. 363, 33–37 (2004).
Johnson, S.W. & North, R.A. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J. Physiol. (Lond.) 450, 455–468 (1992).
Lacey, M.G., Mercuri, N.B. & North, R.A. Actions of cocaine on rat dopaminergic neurones in vitro. Br. J. Pharmacol. 99, 731–735 (1990).
Richardson, N.R. & Roberts, D.C. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 66, 1–11 (1996).
Schoffelmeer, A.N. et al. Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior. J. Neurosci. 31, 1284–1291 (2011).
Paxinos, G. & Watson, C. The Rat Brain in Sterotaxic Coordinates (Academic, New York, 1997).
Brebner, K. et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310, 1340–1343 (2005).
Acknowledgements
The authors would like to thank K. Lee for performing the insulin measurements and K. Haas, B. MacVicar and Y.T. Wang for suggestions on the manuscript. This research was supported by a Canadian Institute of Health Research operating grant (102617) and a Canadian Institute of Health Research new investigator award to S.L.B. G.L. was supported by a Swiss National Science Foundation postdoctoral fellowship. Behavioral experiments done in B.B.'s laboratory were supported by the Swiss National Science Foundation (31003A-133056).
Author information
Authors and Affiliations
Contributions
G.L. and S.L. under supervision of S.L.B. conducted the electrophysiological experiments and wrote the first draft of the manuscript; C.D. and H.Z. under supervision of A.G.P. performed the CPP experiments. G.L. under supervision of B.B. performed the progressive ratio experiments. J.C.Y.W. under supervision of S.L.B. performed the anticipatory behavior experiments. S.K. under supervision of S.M.C. performed the insulin measurements. S.L.B. wrote the manuscript and supervised the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 2666 kb)
Rights and permissions
About this article
Cite this article
Labouèbe, G., Liu, S., Dias, C. et al. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci 16, 300–308 (2013). https://doi.org/10.1038/nn.3321
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3321
This article is cited by
-
Liraglutide restores impaired associative learning in individuals with obesity
Nature Metabolism (2023)
-
G-CuP: the effect of a forced oral glucose intake on alcohol craving and mesolimbic cue reactivity in alcohol dependence—study protocol of a randomized, double-blind, placebo-controlled crossover study
Trials (2022)
-
Neddylation-dependent protein degradation is a nexus between synaptic insulin resistance, neuroinflammation and Alzheimer’s disease
Translational Neurodegeneration (2022)
-
Dietary fructose and its association with the metabolic syndrome in Lebanese healthy adults: a cross-sectional study
Diabetology & Metabolic Syndrome (2022)
-
Insulin modulates emotional behavior through a serotonin-dependent mechanism
Molecular Psychiatry (2022)