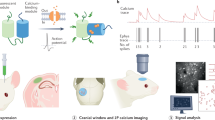
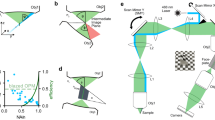
Abstract
Since the introduction of calcium imaging to monitor neuronal activity with single-cell resolution, optical imaging methods have revolutionized neuroscience by enabling systematic recordings of neuronal circuits in living animals. The plethora of methods for functional neural imaging can be daunting to the nonexpert to navigate. Here we review advanced microscopy techniques for in vivo functional imaging and offer guidelines for which technologies are best suited for particular applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
21 June 2017
In the version of this article initially published, the formula for rz_confocal in Box 1 incorrectly had a coefficient of 0.4. The correct coefficient is 1.4. The error has been corrected in the HTML and PDF versions of the article.
21 June 2017
In the version of this article initially published, reference 76 was incorrectly classified as direct wavefront sensing. It should be classified as indirect wavefront sensing. The error has been corrected in the HTML and PDF versions of the article.
References
Crick, F.H.C. Thinking about the brain. Scientific American 241, 219–232 (1979).
Smetters, D., Majewska, A. & Yuste, R. Detecting action potentials in neuronal populations with calcium imaging. Methods 18, 215–221 (1999).
Ahrens, M.B., Orger, M.B., Robson, D.N., Li, J.M. & Keller, P.J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013). Example of whole-brain functional imaging in vivo.
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990). Invention of two-photon microscopy.
Yuste, R. & Denk, W. Dendritic spines as basic functional units of neuronal integration. Nature 375, 682–684 (1995).
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. USA 100, 7319–7324 (2003).
Hasan, M.T. et al. Functional fluorescent Ca2+ indicator proteins in transgenic mice under TET control. PLoS Biol. 2, e163 (2004).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Yuste, R. ed. Imaging: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 2011). Theory and practice of optical imaging methods.
Helmchen, F. & Konnerth, A. ed. Imaging in Neuroscience: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 2011). Review of optical imaging methods in neuroscience.
Lanni, F. & Keller, H.E. in Imaging: A Laboratory Manual (ed. Yuste, R.) 1–56 (Cold Spring Harbor Laboratory Press, 2011).
Ferezou, I. et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 56, 907–923 (2007).
Mohajerani, M.H. et al. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 16, 1426–1435 (2013).
Carandini, M. et al. Imaging the awake visual cortex with a genetically encoded voltage indicator. J. Neurosci. 35, 53–63 (2015).
Pawley, J.B. Handbook of Biological Confocal Microscopy 3rd edn. (Springer, 2006).
Petran, M., Hadravsk, M., Egger, M.D. & Galambos, R. Tandem-scanning reflected-light microscope. J. Opt. Soc. Am. 58, 661–664 (1968).
Mertz, J. Optical sectioning microscopy with planar or structured illumination. Nat. Methods 8, 811–819 (2011).
Chen, B.C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Fahrbach, F.O., Voigt, F.F., Schmid, B., Helmchen, F. & Huisken, J. Rapid 3D light-sheet microscopy with a tunable lens. Opt. Express 21, 21010–21026 (2013).
Tomer, R. et al. SPED light sheet microscopy: fast mapping of biological system structure and function. Cell 163, 1796–1806 (2015).
Quirin, S. et al. Calcium imaging of neural circuits with extended depth-of-field light-sheet microscopy. Opt. Lett. 41, 855–858 (2016).
Bouchard, M.B. et al. Swept confocally-aligned planar excitation (SCAPE) microscopy for high speed volumetric imaging of behaving organisms. Nat. Photonics 9, 113–119 (2015).
Power, R.M. & Huisken, J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 14, 360–373 (2017).
Papagiakoumou, E., de Sars, V., Oron, D. & Emiliani, V. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Opt. Express 16, 22039–22047 (2008).
Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 13, 1468–1476 (2005).
Zhu, G., van Howe, J., Durst, M., Zipfel, W. & Xu, C. Simultaneous spatial and temporal focusing of femtosecond pulses. Opt. Express 13, 2153–2159 (2005).
Papagiakoumou, E., de Sars, V., Emiliani, V. & Oron, D. Temporal focusing with spatially modulated excitation. Opt. Express 17, 5391–5401 (2009).
Schrödel, T., Prevedel, R., Aumayr, K., Zimmer, M. & Vaziri, A. Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nat. Methods 10, 1013–1020 (2013).
Dana, H. et al. Hybrid multiphoton volumetric functional imaging of large-scale bioengineered neuronal networks. Nat. Commun. 5, 3997 (2014).
Nikolenko, V. et al. SLM microscopy: scanless two-photon imaging and photostimulation with spatial light modulators. Front. Neural Circuits 2, 5 (2008).
Rickgauer, J.P., Deisseroth, K. & Tank, D.W. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 17, 1816–1824 (2014).
Hernandez, O. et al. Three-dimensional spatiotemporal focusing of holographic patterns. Nat. Commun. 7, 11928 (2016).
Quirin, S., Peterka, D.S. & Yuste, R. Instantaneous three-dimensional sensing using spatial light modulator illumination with extended depth of field imaging. Opt. Express 21, 16007–16021 (2013).
Quirin, S., Jackson, J., Peterka, D.S. & Yuste, R. Simultaneous imaging of neural activity in three dimensions. Front. Neural Circuits 8, 29 (2014).
Yang, S.J. et al. Extended field-of-view and increased-signal 3D holographic illumination with time-division multiplexing. Opt. Express 23, 32573–32581 (2015).
Broxton, M. et al. Wave optics theory and 3-D deconvolution for the light field microscope. Opt. Express 21, 25418–25439 (2013).
Prevedel, R. et al. Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy. Nat. Methods 11, 727–730 (2014).
Zipfel, W.R., Williams, R.M. & Webb, W.W. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 21, 1369–1377 (2003). Nonlinear microscopies and their applications in biological imaging.
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005). Review of two-photon microscopy.
Briggman, K.L., Helmstaedter, M. & Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471, 183–188 (2011).
Göbel, W. & Helmchen, F. New angles on neuronal dendrites in vivo. J. Neurophysiol. 98, 3770–3779 (2007).
Grewe, B.F., Voigt, F.F., van 't Hoff, M. & Helmchen, F. Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens. Biomed. Opt. Express 2, 2035–2046 (2011).
Beaurepaire, E. & Mertz, J. Epifluorescence collection in two-photon microscopy. Appl. Opt. 41, 5376–5382 (2002).
Yang, W. et al. Simultaneous multi-plane imaging of neural circuits. Neuron 89, 269–284 (2016).
Kong, L. et al. Continuous volumetric imaging via an optical phase-locked ultrasound lens. Nat. Methods 12, 759–762 (2015).
Botcherby, E.J., Juskaitis, R., Booth, M.J. & Wilson, T. An optical technique for remote focusing in microscopy. Opt. Commun. 281, 880–887 (2008).
Anselmi, F., Ventalon, C., Bègue, A., Ogden, D. & Emiliani, V. Three-dimensional imaging and photostimulation by remote-focusing and holographic light patterning. Proc. Natl. Acad. Sci. USA 108, 19504–19509 (2011).
Botcherby, E.J. et al. Aberration-free three-dimensional multiphoton imaging of neuronal activity at kHz rates. Proc. Natl. Acad. Sci. USA 109, 2919–2924 (2012).
Kaplan, A., Friedman, N. & Davidson, N. Acousto-optic lens with very fast focus scanning. Opt. Lett. 26, 1078–1080 (2001).
Duemani Reddy, G., Kelleher, K., Fink, R. & Saggau, P. Three-dimensional random access multiphoton microscopy for functional imaging of neuronal activity. Nat. Neurosci. 11, 713–720 (2008).
Grewe, B.F., Langer, D., Kasper, H., Kampa, B.M. & Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat. Methods 7, 399–405 (2010).
Kirkby, P.A., Nadella, K.M..N.S & Silver, R.A. A compact acousto-optic lens for 2D and 3D femtosecond based 2-photon microscopy. Opt. Express 18, 13720–13744 (2010).
Katona, G. et al. Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Nat. Methods 9, 201–208 (2012).
Tsai, P.S. et al. Ultra-large field-of-view two-photon microscopy. Opt. Express 23, 13833–13847 (2015).
McConnell, G. et al. A novel optical microscope for imaging large embryos and tissue volumes with sub-cellular resolution throughout. eLife 5, e18659 (2016).
Sofroniew, N.J., Flickinger, D., King, J. & Svoboda, K. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. eLife 5, e14472 (2016).
Stirman, J.N., Smith, I.T., Kudenov, M.W. & Smith, S.L. Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain. Nat. Biotechnol. 34, 857–862 (2016).
Cheng, A., Gonçalves, J.T., Golshani, P., Arisaka, K. & Portera-Cailliau, C. Simultaneous two-photon calcium imaging at different depths with spatiotemporal multiplexing. Nat. Methods 8, 139–142 (2011).
Kim, K.H. et al. Multifocal multiphoton microscopy based on multianode photomultiplier tubes. Opt. Express 15, 11658–11678 (2007).
Bewersdorf, J., Pick, R. & Hell, S.W. Multifocal multiphoton microscopy. Opt. Lett. 23, 655–657 (1998).
Watson, B.O. et al. Front. Neurosci. Two-photon microscopy with diffractive optical elements and spatial light modulators. Front. Neurosci. 4, 29 (2010).
Mahou, P. et al. Multicolor two-photon tissue imaging by wavelength mixing. Nat. Methods 9, 815–818 (2012).
Inoue, M. et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat. Methods 12, 64–70 (2015).
Ducros, M., Goulam Houssen, Y., Bradley, J., de Sars, V. & Charpak, S. Encoded multisite two-photon microscopy. Proc. Natl. Acad. Sci. USA 110, 13138–13143 (2013).
Pnevmatikakis, E.A. et al. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron 89, 285–299 (2016).
Prevedel, R. et al. Fast volumetric calcium imaging across multiple cortical layers using sculpted light. Nat. Methods 13, 1021–1028 (2016).
Mukamel, E.A., Nimmerjahn, A. & Schnitzer, M.J. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 63, 747–760 (2009).
Friedrich, J. et al. Multi-scale approaches for high-speed imaging and analysis of large neural populations. Preprint at https://dx.doi.org/10.1101/091132 (2016).
Lu, R. et al. Video-rate volumetric functional imaging of the brain at synaptic resolution. Nat. Neurosci. http://dx.doi.org/10.1038/nn.4516 (2017).
Song, A. et al. Volumetric two-photon imaging of neurons using stereoscopy (vTwINS). Nat. Methods 14, 420–426 (2017).
Podgorski, K. & Ranganathan, G. N. Brain heating induced by near-infrared lasers during multi-photon microscopy. J. Neurophysiol. 116, 1012–1023 (2016).
Horton, N.G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013).
Combs, C.A. et al. Optimizing multiphoton fluorescence microscopy light collection from living tissue by noncontact total emission detection (epiTED). J. Microsc. 241, 153–161 (2011).
Crosignani, V. et al. Deep tissue fluorescence imaging and in vivo biological applications. J. Biomed. Optics 17, 116023 (2012).
Booth, M.J. Adaptive optical microscopy: the ongoing quest for a perfect image. Light-Sci Appl. 3, e165 (2014).
Wang, K. et al. Rapid adaptive optical recovery of optimal resolution over large volumes. Nat. Methods 11, 625–628 (2014).
Ji, N., Milkie, D.E. & Betzig, E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat. Methods 7, 141–147 (2010).
Débarre, D., Booth, M.J. & Wilson, T. Image based adaptive optics through optimisation of low spatial frequencies. Opt. Express 15, 8176–8190 (2007).
Débarre, D. et al. Image-based adaptive optics for two-photon microscopy. Opt. Lett. 34, 2495–2497 (2009).
Neil, M.A.A., Booth, M.J. & Wilson, T. New modal wave-front sensor: a theoretical analysis. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 17, 1098–1107 (2000).
Sun, W., Tan, Z., Mensh, B.D. & Ji, N. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci. 19, 308–315 (2016).
Ji, N. Adaptive optical fluorescence microscopy. Nat. Methods 14, 374–380 (2017).
Ouzounov, D.G. et al. In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain. Nat. Methods 14, 388–390 (2017).
Levene, M.J., Dombeck, D.A., Kasischke, K.A., Molloy, R.P. & Webb, W.W. In vivo multiphoton microscopy of deep brain tissue. J. Neurophysiol. 91, 1908–1912 (2004).
Andermann, M.L. et al. Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron 80, 900–913 (2013).
Low, R.J., Gu, Y. & Tank, D.W. Cellular resolution optical access to brain regions in fissures: imaging medial prefrontal cortex and grid cells in entorhinal cortex. Proc. Natl. Acad. Sci. USA 111, 18739–18744 (2014).
Attardo, A., Fitzgerald, J.E. & Schnitzer, M.J. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature 523, 592–596 (2015).
Ghosh, K.K. et al. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878 (2011).
Ziv, Y. et al. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 16, 264–266 (2013).
Flusberg, B.A. et al. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat. Methods 5, 935–938 (2008).
Szabo, V., Ventalon, C., De Sars, V., Bradley, J. & Emiliani, V. Spatially selective holographic photoactivation and functional fluorescence imaging in freely behaving mice with a fiberscope. Neuron 84, 1157–1169 (2014).
Helmchen, F., Fee, M.S., Tank, D.W. & Denk, W. A miniature head-mounted two-photon microscope. high-resolution brain imaging in freely moving animals. Neuron 31, 903–912 (2001).
Flusberg, B.A. et al. Fiber-optic fluorescence imaging. Nat. Methods 2, 941–950 (2005).
Myaing, M.T., MacDonald, D.J. & Li, X. Fiber-optic scanning two-photon fluorescence endoscope. Opt. Lett. 31, 1076–1078 (2006).
Rivera, D.R. et al. Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue. Proc. Natl. Acad. Sci. USA 108, 17598–17603 (2011).
Göbel, W., Kerr, J.N.D., Nimmerjahn, A. & Helmchen, F. Miniaturized two-photon microscope based on a flexible coherent fiber bundle and a gradient-index lens objective. Opt. Lett. 29, 2521–2523 (2004).
Chen, Z., Wei, L., Zhu, X. & Min, W. Extending the fundamental imaging-depth limit of multi-photon microscopy by imaging with photo-activatable fluorophores. Opt. Express 20, 18525–18536 (2012).
Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6, 2 (2013).
Theis, L. et al. Benchmarking spike rate inference in population calcium imaging. Neuron 90, 471–482 (2016).
Insel, T.R., Landis, S.C. & Collins, F.S. The NIH BRAIN Initiative. Research priorities. Science 340, 687–688 (2013).
Yuste, R. & Bargmann, C. Towards a global BRAIN Initiative. Cell http://dx.doi.org/10.1016/j.cell.2017.02.023 (2017).
Robertson, M. Biology in the 1980s, plus or minus a decade. Nature 285, 358–359 (1980).
Acknowledgements
The authors thank D. Peterka and other members of R.Y.'s lab for fruitful discussions. W.Y. holds a Career Award at the Scientific Interface from Burroughs Wellcome Fund. Our work is supported by the National Eye Institute (NEI) under grants number DP1EY024503, R01EY011787 (R.Y.); National Institute of Mental Health (NIMH) under grants numbers R01MH101218, R01MH100561 (R.Y.) and the Defense Advanced Research Projects Agency (DARPA) under contracts number N66001-15-C-4032 (SIMPLEX) (R.Y.) and HR0011-17-C-0026 (R.Y.). This material is based upon work supported by, or in part by, the US Army Research Laboratory and the US Army Research Office under contract number W911NF-12-1-0594 (MURI) (R.Y.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors have patent applications related to holographic microscopy.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 (PDF 201 kb)
Rights and permissions
About this article
Cite this article
Yang, W., Yuste, R. In vivo imaging of neural activity. Nat Methods 14, 349–359 (2017). https://doi.org/10.1038/nmeth.4230
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4230
This article is cited by
-
Mesoscopic calcium imaging in a head-unrestrained male non-human primate using a lensless microscope
Nature Communications (2024)
-
Video-based pooled screening yields improved far-red genetically encoded voltage indicators
Nature Methods (2023)
-
A mosquito mouthpart-like bionic neural probe
Microsystems & Nanoengineering (2023)
-
Transparent neural implantable devices: a comprehensive review of challenges and progress
npj Flexible Electronics (2022)
-
Deep learning autofluorescence-harmonic microscopy
Light: Science & Applications (2022)