Abstract
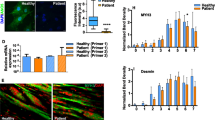
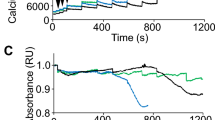
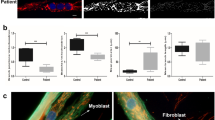
The integrity of the plasma membrane is maintained through an active repair process, especially in skeletal and cardiac muscle cells, in which contraction-induced mechanical damage frequently occurs in vivo1,2. Muscular dystrophies (MDs) are a group of muscle diseases characterized by skeletal muscle wasting and weakness3,4. An important cause of these group of diseases is defective repair of sarcolemmal injuries, which normally requires Ca2+ sensor proteins5,6,7,8 and Ca2+-dependent delivery of intracellular vesicles to the sites of injury8,9. MCOLN1 (also known as TRPML1, ML1) is an endosomal and lysosomal Ca2+ channel whose human mutations cause mucolipidosis IV (ML4), a neurodegenerative disease with motor disabilities10,11. Here we report that ML1-null mice develop a primary, early-onset MD independent of neural degeneration. Although the dystrophin-glycoprotein complex and the known membrane repair proteins are expressed normally, membrane resealing was defective in ML1-null muscle fibers and also upon acute and pharmacological inhibition of ML1 channel activity or vesicular Ca2+ release. Injury facilitated the trafficking and exocytosis of vesicles by upmodulating ML1 channel activity. In the dystrophic mdx mouse model, overexpression of ML1 decreased muscle pathology. Collectively, our data have identified an intracellular Ca2+ channel that regulates membrane repair in skeletal muscle via Ca2+-dependent vesicle exocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Clarke, M.S., Khakee, R. & McNeil, P.L. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J. Cell Sci. 106, 121–133 (1993).
McNeil, P.L. & Khakee, R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 140, 1097–1109 (1992).
Davies, K.E. & Nowak, K.J. Molecular mechanisms of muscular dystrophies: old and new players. Nat. Rev. Mol. Cell Biol. 7, 762–773 (2006).
Rahimov, F. & Kunkel, L.M. The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J. Cell Biol. 201, 499–510 (2013).
Bansal, D. et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423, 168–172 (2003).
Chakrabarti, S. et al. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J. Cell Biol. 162, 543–549 (2003).
Barresi, R. et al. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat. Med. 10, 696–703 (2004).
McNeil, P. Membrane repair redux: redox of MG53. Nat. Cell Biol. 11, 7–9 (2009).
Cai, C. et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 11, 56–64 (2009).
Sun, M. et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 9, 2471–2478 (2000).
Venugopal, B. et al. Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. Am. J. Hum. Genet. 81, 1070–1083 (2007).
Dong, X.P. et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455, 992–996 (2008).
Shen, D. et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 3, 731 (2012).
Samie, M. et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev. Cell 26, 511–524 (2013).
de Figueiredo, P. & Brown, W.J. A role for calmodulin in organelle membrane tubulation. Mol. Biol. Cell 6, 871–887 (1995).
Straub, V., Rafael, J.A., Chamberlain, J.S. & Campbell, K.P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139, 375–385 (1997).
Weitz, R. et al. Muscle involvement in mucolipidosis IV. Brain Dev. 12, 524–528 (1990).
Zlotogora, J., Ben Ezra, D., Livni, N., Ashkenazi, A. & Cohen, T. A muscle disorder as presenting symptom in a child with mucolipidosis IV. Neuropediatrics 14, 104–105 (1983).
Venugopal, B. et al. Chaperone-mediated autophagy is defective in mucolipidosis type IV. J. Cell. Physiol. 219, 344–353 (2009).
Abe, A. et al. Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J. Clin. Invest. 105, 1563–1571 (2000).
Campbell, K.P. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell 80, 675–679 (1995).
Cai, C. et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J. Biol. Chem. 284, 15894–15902 (2009).
Bansal, D. & Campbell, K.P. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 14, 206–213 (2004).
Weisleder, N. et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci. Transl. Med. 4, 139ra185 (2012).
Idone, V. et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 180, 905–914 (2008).
Hua, Z. et al. v-SNARE composition distinguishes synaptic vesicle pools. Neuron 71, 474–487 (2011).
Reddy, A., Caler, E.V. & Andrews, N.W. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106, 157–169 (2001).
Corrotte, M. et al. Caveolae internalization repairs wounded cells and muscle fibers. Elife 2, e00926 (2013).
Jimenez, A.J. et al. ESCRT machinery is required for plasma membrane repair. Science 343, 1247136 (2014).
Demonbreun, A.R. et al. Impaired muscle growth and response to insulin-like growth factor 1 in dysferlin-mediated muscular dystrophy. Hum. Mol. Genet. 20, 779–789 (2011).
Bolsover, F.E., Murphy, E., Cipolotti, L., Werring, D.J. & Lachmann, R.H. Cognitive dysfunction and depression in Fabry disease: a systematic review. J. Inherit. Metab. Dis. 37, 177–187 (2014).
Springer, M.L., Rando, T.A. & Blau, H.M. Gene delivery to muscle. Curr. Protoc. Hum. Genet. 31, 13.14 (2002).
Dong, X.P. et al. PI3,5P2 controls membrane traffic by direct activation of mucolipin Ca release channels in the endolysosome. Nat. Commun. 1, 38 (2010).
Wang, X. et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151, 372–383 (2012).
Brooke, M.H. & Kaiser, K.K. Muscle fiber types: how many and what kind? Arch. Neurol. 23, 369–379 (1970).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants (NS062792, MH096595 and AR060837 to H.X.; HL116546 and AR064241 to R.H.). We are grateful to S. Slaugenhaupt for the ML1 KO mice, L. Looger for the GCaMP3 construct, R. Edwards for the Vamp7-pHluorin construct, the Center for Live-Cell Imaging at the University of Michigan for the help on TIRF Imaging, and R. Hume and M. Akaaboune for comments on the manuscript. We appreciate the encouragement and helpful comments of other members of the Xu laboratory.
Author information
Authors and Affiliations
Contributions
X.C. initiated the project; H.X., X.C., J.D. and R.H. designed the research; X.C., X.Z., Q.G., M.A.S., M.A., W.L.T., Y.Z. and L.D. performed the research; N.S., X.L., A.G.G., X.W., M.F. and L.X. contributed the new reagents; X.C., X.Z., Q.G., M.A.S., M.A., W.L.T., J.D., R.H. and H.X. analyzed the data; H.X. and X.C. wrote the paper with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 6007 kb)
Supplementary Data Set
Source data for Supplementary Figures. (XLSX 30 kb)
SLO treatment induced exocytosis of ML1- and Vamp7-pHluorin-positive vesicles (related to Supplementary Fig. 6a).
Vesicle fusion with the plasma membrane was visualized using pHluorin-based TIRF imaging upon SLO (0.7 g/ml) treatment. Scale bar = 10 μm. (AVI 5560 kb)
Rights and permissions
About this article
Cite this article
Cheng, X., Zhang, X., Gao, Q. et al. The intracellular Ca2+ channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med 20, 1187–1192 (2014). https://doi.org/10.1038/nm.3611
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3611
This article is cited by
-
Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology
Nature Reviews Molecular Cell Biology (2024)
-
Biological activity reduction and mitochondrial and lysosomal dysfunction of mesenchymal stem cells aging in vitro
Stem Cell Research & Therapy (2022)
-
The cellular response to plasma membrane disruption for nanomaterial delivery
Nano Convergence (2022)
-
Plasma membrane integrity: implications for health and disease
BMC Biology (2021)
-
Estradiol analogs attenuate autophagy, cell migration and invasion by direct and selective inhibition of TRPML1, independent of estrogen receptors
Scientific Reports (2021)