Abstract
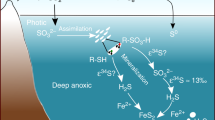
The ability of microbes to metabolize arsenic may have emerged more than 3.4 billion years ago1,2. Some of the modern environments in which prominent arsenic metabolism occurs are anoxic3,4, as were the Precambrian oceans. Early oceans may also have had a relatively high abundance of arsenic5. However, it is unclear whether arsenic cycling occurred in ancient environments. Here we assess the chemistry and nature of cell-like globules identified in salt-encrusted portions of 2.72-billion-year-old fossil stromatolites from Western Australia. We use Raman spectroscopy and X-ray fluorescence to show that the globules are composed of organic carbon and arsenic (As). We argue that our data are best explained by the occurrence of a complete arsenic cycle at this site, with As(III) oxidation and As(V) reduction by microbes living in permanently anoxic conditions. We therefore suggest that arsenic cycling could have occurred more widely in marine environments in the several hundred million years before the Earth’s atmosphere and shallow oceans were oxygenated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lebrun, E. et al. Arsenite oxidase, an ancient bioenergetic enzyme. Mol. Biol. Evol. 20, 686–693 (2003).
Duval, S., Ducluzeau, A-L., Nitschke, W. & Schoepp-Cothenet, B. Enzyme phylogenies as markers for the oxidation state of the environment: The case of respiratory arsenate reductase and related enzymes. BMC Evol. Biol. 8, 206–219 (2008).
Kulp, T. R. et al. Arsenic (III) fuels anoxygenic photosynthesis in hot springs biofilms from Mono Lake, California. Science 321, 967–970 (2008).
Oremland, R. S. et al. Anaerobic oxidation of arsenite in Mono Lake water and by a facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl. Environ. Microbiol. 68, 4795–4802 (2002).
Bergman, I. A. & Kolesov, G. M. Arsenic, antimony, and bismuth as indicators of the genesis of ore material in Early Precambrian ferrous quartzite formations. Geochem. Int. 50, 816–831 (2012).
Philippot, P. et al. Early traces of life investigations in drilling Archean hydrothermal and sedimentary rocks of the Pilbara Craton, Western Australia and Barberton Greenstone Belt, South Africa. C. R. Palevol 8, 649–663 (2009).
Lepot, K., Benzerara, K., Brown, G. E. Jr & Philippot, P. Microbially influenced formation of 2.724-million-year-old stromatolites. Nature Geosci. 11, 18–121 (2008).
Lepot, K. et al. Organic matter heterogeneities in 2.72 Ga stromatolites: Alteration versus preservation by sulfur incorporation. Geochim. Cosmochim. Acta 73, 6579–6599 (2009).
Buick, R. The antiquity of oxygenic photosynthesis: Evidence from stromatolites in sulphate-deficient Archaean lakes. Science 255, 74–77 (1992).
Bolhar, R. & Van Kranendonk, M. J. A non-marine depositional setting for the northern Fortescue Group, Pilbara Craton, inferred from trace element geochemistry of stromatolitic carbonates. Precambr. Res. 155, 229–250 (2007).
Stolz, J. F., Basu, P., Santini, J. M. & Oremland, R. S. Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 60, 107–130 (2006).
Braissant, O. et al. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 5, 401–411 (2007).
Buick, R. When did oxygenic photosynthesis evolve? Phil. Trans. R. Soc. Lond. 363, 2731–2743 (2008).
Hayes, J. M. in Early Life on Earth (ed Bengston, S.) 220–236 (Columbia Univ. Press, 1994).
Eigenbrode, J. L. & Freeman, K. H. Late Archean rise of aerobic microbial ecosystems. Proc. Natl Acad. Sci. USA 103, 15759–15764 (2006).
Hinrichs, K. U. Microbial fixation of methane carbon at 2.7 Ga: Was an anaerobic mechanism possible? Geochem. Geophys. Geosyst. 3, 1–10 (2002).
Thomazo, C., Ader, M., Farquhar, J. & Philippot, P. Methanotrophs regulated atmospheric sulfur isotope anomalies during the Mesoarchean (Tumbiana Formation, Western Australia). Earth Planet. Sci. Lett. 279, 65–75 (2009).
Thomazo, C., Ader, M. & Philippot, P. Extreme 15N-enrichments in 2.72-Gyr-old sediments: Evidence for a turning point in the nitrogen cycle. Geobiology 9, 107–120 (2011).
Griffin, B. M., Schott, J. & Schink, B. Nitrite, an electron donor for anoxygenic photosynthesis. Science 316, 1870 (2007).
Schoepp-Cothenet, B. et al. Menaquinone as pool quinone in a purple bacterium. Proc. Natl Acad. Sci. USA 106, 8549–8554 (2009).
Oremland, R. S., Saltikov, C. W., Wolfe-Simon, F. & Stolz, J. F. Arsenic in the evolution of Earth and extraterrestrial ecosystems. Geomicrobiol. J. 26, 522–536 (2009).
Oremland, R. S., Stolz, J. F. & Hollibaugh, J. T. The microbial arsenic cycle in Mono Lake, California. FEMS Microbiol. Ecol. 48, 15–27 (2004).
Hoeft, S. E., Kulp, T. R., Han, S., Lanoil, B. & Oremland, R. S. Coupled arsenotrophy in a hot spring photosynthetic biofilm at Mono Lake, California. Appl. Environ. Microbiol. 76, 4633–4639 (2010).
Van Lis, R., Nitschke, W., Duval, S. & Schoepp-Cothenet, B. Arsenics as bioenergetic substrates. Biochim. Biophys. Acta 1827, 176–188 (2013).
Oremland, R. S. et al. A microbial arsenic cycle in a salt-saturated, extreme environment. Science 308, 1305–1308 (2005).
Miller, L. G., Jellison, R., Oremland, R. S. & Culbertson, C. W. Meromixis in hypersaline Mono Lake, California. 3. Biogeochemical response to stratification and overturn. Limnol. Oceanogr. 38, 1040–1051 (1993).
Farías, M. E. et al. The discovery of stromatolites developing at 3570 m above sea level in a high-altitude volcanic lake Socompa, Argentinean Andes. PLoS ONE 8, e53497 (2013).
Lara, J. et al. Enrichment of arsenic transforming and resistant heterotrophic bacteria from sediments of two salt lakes in Northern Chile. Extremophiles 16, 523–538 (2012).
Acknowledgements
The authors are grateful to D. Paterson, M. De Jonge (AS), C. Ryan (CSIRO), D. Grolimund, C. Borca (SLS) and F. Segura-Ruiz (ESRF) for their help during SR-XRF experiments. We thank the Institut de Physique du Globe de Paris and the Geological Survey of Western Australia for supporting the PDP. This work was supported by a grant from the Agence Nationale de la Recherche project ‘eLIFE2’ to P.P. and the UnivEarths Labex programme at Sorbonne Paris Cité (ANR-10-LABX-0023 and ANR-11-IDEX-0005-02). This is IPGP contribution number 3559.
Author information
Authors and Affiliations
Contributions
M.C.S., A.S., P.P., M.A.v.Z. and K.M. carried out the synchrotron and Raman experiments and treated the data. P.P., M.C.S. and A.S. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 64427 kb)
Rights and permissions
About this article
Cite this article
Sforna, M., Philippot, P., Somogyi, A. et al. Evidence for arsenic metabolism and cycling by microorganisms 2.7 billion years ago. Nature Geosci 7, 811–815 (2014). https://doi.org/10.1038/ngeo2276
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2276
This article is cited by
-
Unexpected genetic and microbial diversity for arsenic cycling in deep sea cold seep sediments
npj Biofilms and Microbiomes (2023)
-
Towards routine 3D characterization of intact mesoscale samples by multi-scale and multimodal scanning X-ray tomography
Scientific Reports (2022)
-
Gulf of Mexico blue hole harbors high levels of novel microbial lineages
The ISME Journal (2021)
-
Phosphate-Arsenic Interactions in Halophilic Microorganisms of the Microbial Mat from Laguna Tebenquiche: from the Microenvironment to the Genomes
Microbial Ecology (2021)
-
Genomic insights into an andean multiresistant soil actinobacterium of biotechnological interest
World Journal of Microbiology and Biotechnology (2021)