Abstract
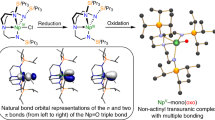
Studies of transuranic organometallic complexes provide a particularly valuable insight into covalent contributions to the metal–ligand bonding, in which the subtle differences between the transuranium actinide ions and their lighter lanthanide counterparts are of fundamental importance for the effective remediation of nuclear waste. Unlike the organometallic chemistry of uranium, which has focused strongly on UIII and has seen some spectacular advances, that of the transuranics is significantly technically more challenging and has remained dormant. In the case of neptunium, it is limited mainly to NpIV. Here we report the synthesis of three new NpIII organometallic compounds and the characterization of their molecular and electronic structures. These studies suggest that NpIII complexes could act as single-molecule magnets, and that the lower oxidation state of NpII is chemically accessible. In comparison with lanthanide analogues, significant d- and f-electron contributions to key NpIII orbitals are observed, which shows that fundamental neptunium organometallic chemistry can provide new insights into the behaviour of f-elements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nash, K., Madic, C., Mathur, J. N. & Lacquement, J. in The Chemistry of the Actinide and Transactinide Elements Vol. 5 (eds Katz, J. J. et al.) Ch. 25, 2799–2910 (Springer, 2010).
LaPierre, H. S. & Meyer, K. Activation of small molecules by molecular uranium complexes. Progr. Inorg. Chem. 58, 303–416 (2014).
Liddle, S. T. The renaissance of non-aqueous uranium chemistry. Angew. Chem. Int. Ed. 54, 8604–8641 (2015).
Arnold, P. L., McMullon, M. W., Rieb, J. & Kuhn, F. E. C–H bond activation by f-block complexes. Angew. Chem. Int. Ed. 54, 82–100 (2015).
Fischer, E. O., Laubereau, P., Baumgärtner, F. & Kanellakopulos, B. Tricyclopentadienylneptunium-chlorid. J. Organomet. Chem. 5, 583–584 (1966).
Baumgärtner, F., Fischer, E. O., Kanellakopulos, B. & Laubereau, P. Tetrakis(cyclopentadienyl)neptunium(IV). Angew. Chem. 80, 661–661 (1968).
Bohlander, R. The Organometallic Chemistry of Neptunium (Kernforschungszentrum Karlsruhe GmbH, 1986).
De Ridder, D. J. A., Rebizant, J., Apostolidis, C., Kanellakopulos, B. & Dornberger, E. Bis(cyclooctatetraenyl)neptunium(IV). Acta Cryst. C 52, 597–600 (1996).
Magnani, N. et al. Magnetic memory effect in a transuranic mononuclear complex. Angew. Chem. Int. Ed. 50, 1696–1698 (2011).
Eisenberg, D. C., Streitwieser, A. & Kot, W. K. Electron transfer in organouranium and transuranium systems. Inorg. Chem. 29, 10–14 (1990).
Burns, C. & Eisen, M. in The Chemistry of the Actinide and Transactinide Elements Vol. 5 (eds Katz, J. J. et al.) Ch. 25, 2799–2910 (Springer, 2010).
Arnold, P. L., Mansell, S. M., Maron, L. & McKay, D. Spontaneous reduction and C–H borylation of arenes mediated by uranium(III) disproportionation. Nature Chem. 4, 668–674 (2012).
La Pierre, H. S., Scheurer, A., Heinemann, F. W., Hieringer, W. & Meyer, K. Synthesis and characterization of a uranium(II) monoarene complex supported by δ backbonding. Angew. Chem. Int. Ed. 53, 7158–7162 (2014).
Hong, G., Schautz, F. & Dolg, M. Ab initio study of metal−ring bonding in the bis(η6-benzene)lanthanide and -actinide complexes M(C6H6)2 (M = La, Ce, Nd, Gd, Tb, Lu, Th, U). J. Am. Chem. Soc. 121, 1502–1512 (1999).
Sessler, J. L., Cho, W.-S., Lynch, V. & Král, V. Missing-link macrocycles: hybrid heterocalixarene analogues formed from several different building blocks. Chem. Eur. J. 8, 1134–1143 (2002).
Arnold, P. L. et al. Switchable π-coordination and C–H metallation in small-cavity macrocyclic uranium and thorium complexes. Chem. Sci. 5, 756–765 (2014).
Avens, L. R. et al. A convenient entry into trivalent actinide chemistry: synthesis and characterization of AnI3(THF)4 and An[N(SiMe3)2]3 (An = U, Np, Pu). Inorg. Chem. 33, 2248–2256 (1994).
Arnold, P. L. et al. Synthesis of bimetallic uranium and neptunium complexes of a binucleating macrocycle and determination of the solid-state structure by magnetic analysis. Inorg. Chem. 49, 5341–5343 (2010).
Bursten, B. E., Rhodes, L. F. & Strittmatter, R. J. The bonding in tris(η5-cyclopentadienyl)actinide complexes. IV: electronic structural effects in AnCl3 and (η5-C5H5)3An (An = thorium–californium) complexes. J. Less-Common Met. 149, 207–211 (1989).
Ilango, S., Vidjayacoumar, B. & Gambarotta, S. Samarium complexes of a σ-/π-pyrrolide/arene based macrocyclic ligand. Dalton Trans. 39, 6853–6857 (2010).
Arnold, P. L., Farnaby, J. H., Gardiner, M. G. & Love, J. B. Uranium(III) coordination chemistry and oxidation in a flexible small-cavity macrocycle. Organometallics 34, 2114–2117 (2015).
Shannon, R. D. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 32, 751–767 (1976).
Minasian, S. G. et al. Synthesis and structure of (Ph4P)2MCl6 (M = Ti, Zr, Hf, Th, U, Np, Pu). Inorg. Chem. 51, 5728–5736 (2012).
Bagnall, K. W., Payne, G. F., Alcock, N. W., Flanders, D. J. & Brown, D. Actinide structural studies. Part 8. Some new oxygen-donor complexes of trichloro(cyclopentadienyl)neptunium(IV); the crystal structure of trichloro(η5-cyclopentadienyl)bis(methyldiphenylphosphine oxide)neptunium(IV). Dalton Trans. 783–787 (1986).
Langeslay, R. R., Fieser, M. E., Ziller, J. W., Furche, F. & Evans, W. J. Synthesis, structure, and reactivity of crystalline molecular complexes of the {[C5H3(SiMe3)2]3Th}1– anion containing thorium in the formal +2 oxidation state. Chem. Sci. 6, 517–521 (2015).
MacDonald, M. R. et al. Identification of the +2 oxidation state for uranium in a crystalline molecular complex, [K(2.2.2-cryptand)][(C5H4SiMe3)3U]. J. Am. Chem. Soc. 135, 13310–13313 (2013).
Blake, P. C., Lappert, M. F., Atwood, J. L. & Zhang, H. The synthesis and characterisation, including X-ray diffraction study, of [Th{η-C5H3(SiMe3)2}3]; the first thorium(III) crystal structure. Chem. Comm. 1148–1149 (1986).
De Ridder, D. J. A., Apostolidis, C., Rebizant, J., Kanellakopulos, B. & Maier, R. Tris(η5-cyclopentadienyl)phenolatoneptunium(IV). Acta Cryst. C 52, 1436–1438 (1996).
Arnold, P. L. et al. Strongly coupled binuclear uranium–oxo complexes from uranyl oxo rearrangement and reductive silylation. Nature Chem. 4, 221–227 (2012).
Meihaus, K. R. & Long, J. R. Actinide-based single-molecule magnets. Dalton Trans. 44, 2517–2528 (2015).
Rinehart, J. D. & Long, J. R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2, 2078–2085 (2011).
Moro, F., Mills, D. P., Liddle, S. T. & van Slageren, J. The inherent single-molecule magnet character of trivalent uranium. Angew. Chem. Int. Ed. 52, 3430–3433 (2013).
Kaltsoyannis, N. & Scott, P. The f Elements (Oxford Univ. Press, 1999).
Prodan, I. D., Scuseria, G. E. & Martin, R. L. Covalency in the actinide dioxides: systematic study of the electronic properties using screened hybrid density functional theory. Phys. Rev. B 76, 033101 (2007).
Kirker, I. & Kaltsoyannis, N. Does covalency really increase across the 5f series? A comparison of molecular orbital, natural population, spin and electron density analyses of AnCp3 (An = Th–Cm; Cp = η5-C5H5). Dalton Trans. 40, 124–131 (2011).
Neidig, M. L., Clark, D. L. & Martin, R. L. Covalency in f-element complexes. Coord. Chem. Rev. 257, 394–406 (2013).
Huang, Q.-R., Kingham, J. R. & Kaltsoyannis, N. The strength of actinide-element bonds from the quantum theory of atoms-in-molecules. Dalton Trans. 44, 2554–2566 (2015).
Acknowledgements
Experiments were supported by the European FP7 TALISMAN project, under contract with the European Commission, the JRC ITU USERLAB programme, the University of Edinburgh, European Cooperation in Science and Technology action CM1006 and the Engineering and Physical Sciences Research Council EP/H004823/1 and EP/M010554/1. M.S.D. acknowledges the European Commission for support in the frame of the Training and Mobility of Researchers programme. We thank G. Nichol for help with the X-ray crystallography, A. Morgenstern for help with data collection and the Organometallic Chemistry Laboratory, CENT, University of Warsaw, for additional data collection. N.K. thanks the UCL High Performance Computing Facility (Legion@UCL), the University of Manchester's computational shared facility and associated support staff and services.
Author information
Authors and Affiliations
Contributions
M.S.D. synthesized and purified the compounds and collected and analysed synthetic data; J.H.F. provided ligand samples and analysed synthetic data; C.A. collected and analysed synthetic data; E.C. collected and analysed magnetic data; O.W. collected and analysed crystallographic data; N.M. analysed magnetic data; M.G.G. provided ligand samples; J.B.L. designed the study, analysed the data and wrote the manuscript; N.K. designed the computational study, carried out and analysed the calculations and wrote the manuscript; R.C. designed the study, analysed the data and wrote the manuscript; P.L.A. designed the study, analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1751 kb)
Supplementary information
Crystallographic data for compound 1. (CIF 1408 kb)
Supplementary information
Crystallographic data for compound 2. (CIF 665 kb)
Supplementary information
Crystallographic data for compound 3. (CIF 280 kb)
Rights and permissions
About this article
Cite this article
Dutkiewicz, M., Farnaby, J., Apostolidis, C. et al. Organometallic neptunium(III) complexes. Nature Chem 8, 797–802 (2016). https://doi.org/10.1038/nchem.2520
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2520
This article is cited by
-
A terminal neptunium(V)–mono(oxo) complex
Nature Chemistry (2022)
-
Isolation and characterization of a californium metallocene
Nature (2021)
-
Carbon oxygenate transformations by actinide compounds and catalysts
Nature Reviews Chemistry (2017)
-
Actinide covalency measured by pulsed electron paramagnetic resonance spectroscopy
Nature Chemistry (2017)
-
Thoroughly enthralling thulium
Nature Chemistry (2017)