Abstract
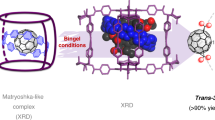
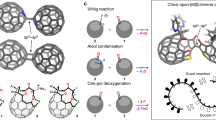
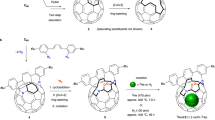
The water dimer is an ideal chemical species with which to study hydrogen bonds. Owing to the equilibrium between the monomer and oligomer structure, however, selective generation and separation of a genuine water dimer has not yet been achieved. Here, we report a synthetic strategy that leads to the successful encapsulation of one or two water molecules inside fullerene C70. These endohedral C70 compounds offer the opportunity to study the intrinsic properties of a single water molecule without any hydrogen bonding, as well as an isolated water dimer with a single hydrogen bond between the two molecules. The unambiguously determined off-centre position of water in (H2O)2@C70 by X-ray diffraction provides insights into the formation of (H2O)2@C70. Subsequently, the 1H NMR spectroscopic measurements for (H2O)2@C70 confirmed the formation of a single hydrogen bond rapidly interchanging between the encapsulated water dimer. Our theoretical calculations revealed a peculiar cis-linear conformation of the dimer resulting from confinement effects inside C70.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Keutsch, F. N. & Saykally, R. J. Water clusters: untangling the mysteries of the liquid, one molecule at a time. Proc. Natl Acad. Sci. USA 98, 10533–10540 (2001).
Ludwig, R. Water: from clusters to the bulk. Angew. Chem. Int. Ed. 40, 1808–1827 (2001).
Mukhopadhyay, A., Cole, W. T. S. & Saykally, R. J. Chem. Phys. Lett. 633, 13–26 (2015).
Morokuma, K. & Pedersen, L. Molecular-orbital studies of hydrogen bonds. An ab initio calculation for dimeric H2O. J. Chem. Phys. 48, 3275–3282 (1968).
Feller, D. Application of systematic sequences of wave functions to the water dimer. J. Chem. Phys. 96, 6104–6114 (1992).
Xu, X. & Goddard III, W. A. Bonding properties of the water dimer: a comparative study of density functional theories. J. Phys. Chem. A 108, 2305–2313 (2004).
Vaida, V. Perspective: water cluster mediated atmospheric chemistry. J. Chem. Phys. 135, 020901 (2011).
Tretyakov, M. Y., Serov, E. A., Koshelev, M. A., Parshin, V. V. & Krupnov, A. F. Water dimer rotationally resolved millimeter-wave spectrum observation at room temperature. Phys. Rev. Lett. 110, 093001 (2013).
Ong, W. Q. et al. Encapsulation of conventional and unconventional water dimers by water-binding foldamers. Org. Lett. 13, 3194–3197 (2011).
Liang, J. et al. A 3D 12-ring zeolite with ordered 4-ring vacancies occupied by (H2O)2 dimers. Chem. Eur. J. 20, 16097–16101 (2014).
Buchanan, E. G. & Zwier, T. S. Binding water clusters to an aromatic-rich hydrophobic pocket: [2.2.2]paracyclophane–(H2O)n, n = 1–5. J. Phys. Chem. A 118, 8583–8596 (2014).
Yoshizawa, M. et al. Endohedral clusterization of ten water molecules into a ‘molecular ice’ within the hydrophobic pocket of a self-assembled cage. J. Am. Chem. Soc. 127, 2798–2799 (2005).
Maniwa, Y. et al. Water-filled single-wall carbon nanotubes as molecular nanovalves. Nature Mater. 6, 135–141 (2007).
Kurotobi, K. & Murata, Y. A single molecule of water encapsulated in fullerene C60 . Science 333, 613–616 (2011).
Akasaka, T. & Nagase, S. Endofullerenes: A New Family of Carbon Clusters (Kluwer Academic, 2002).
Lu, X., Feng, L., Akasaka, T. & Nagase, S. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 41, 7723–7760 (2012).
Murphy, T. A. et al. Observation of atomlike nitrogen in nitrogen-implanted solid C60 . Phys. Rev. Lett. 77, 1075 (1996).
Aoyagi, S. et al. A layered ionic crystal of polar Li@C60 superatoms. Nature Chem. 2, 678–683 (2010).
Nikawa, H. et al. The effect of atomic nitrogen on the C60 cage. Chem. Commun. 46, 631–633 (2010).
Suetsuna, T. et al. Separation of N2@C60 and N@C60 . Chem. Eur. J. 8, 5079–5083 (2002).
Komatsu, K. & Murata, Y. A new route to an endohedral fullerene by way of σ-framework transformations. Chem. Lett. 34, 886–891 (2005).
Murata, M., Murata, Y. & Komatsu, K. Surgery of fullerenes. Chem. Commun. 6083–6094 (2008).
Murata, M., Murata, Y. & Komatsu, K. in Organic Nanomaterials (eds Torres, T. & Bottari, G.) 225–240 (Wiley-Blackwell, 2013).
Komatsu, K., Murata, M. & Murata, Y. Encapsulation of molecular hydrogen in fullerene C60 by organic synthesis. Science 307, 238–240 (2005).
Murata, M., Murata, Y. & Komatsu, K. Synthesis and properties of endohedral C60 encapsulating molecular hydrogen. J. Am. Chem. Soc. 128, 8024–8033 (2006).
Morinaka, Y., Tanabe, F., Murata, M., Murata, Y. & Komatsu, K. Rational synthesis, enrichment, and 13C NMR spectra of endohedral C60 and C70 encapsulating a helium atom. Chem. Commun. 46, 4532–4534 (2010).
Khong, A. et al. An NMR study of He2 inside C70 . J. Am. Chem. Soc. 120, 6380–6383 (1998).
Peres, T. et al. Some new diatomic molecule containing endohedral fullerenes. Int. J. Mass Spectrom. 210/211, 241–247 (2001).
Morinaka, Y. et al. X-ray observation of a helium atom and placing a nitrogen atom inside He@C60 and He@C70 . Nature Commun. 4, 1554 (2013).
Murata, Y., Maeda, S., Murata, M. & Komatsu, K. Encapsulation and dynamic behavior of two H2 molecules in an open-cage C70 . J. Am. Chem. Soc. 130, 6702–6703 (2008).
Murata, M., Maeda, S., Morinaka, Y., Murata, Y. & Komatsu, K. Synthesis and reaction of fullerene C70 encapsulating two molecules of H2 . J. Am. Chem. Soc. 130, 15800–15801 (2008).
Vougioukalakis, G. C., Roubelakis, M. M. & Orfanopoulos, M. Open-cage fullerenes: towards the construction of nanosized molecular containers. Chem. Soc. Rev. 39, 817–844 (2010).
Gan, L., Yang, D., Zhang, Q. & Huang, H. Preparation of open-cage fullerenes and incorporation of small molecules through their orifices. Adv. Mater. 22, 1498–1507 (2010).
Herrmann, A., Diederich, F., Thilgen, C., Meer, H. U. & Müller, W. H. Chemistry of the higher fullerenes: preparative isolation of C76 by HPLC and synthesis, separation, and characterization of Diels–Alder monoadducts of C70 and C76 . Helv. Chim. Acta 77, 1689–1704 (1994).
Thilgen, C. & Diederich, F. Structural aspects of fullerene chemistry—a journey through fullerene chirality. Chem. Rev. 106, 5049–5135 (2006).
Hirsch, A. & Brettreich, M. Fullerenes: Chemistry and Reactions (Wiley-VCH, 2005).
Williams, M., Tummala, N. R., Aziz, S. G., Risko, C. & Bŕedas, J.-L. Influence of molecular shape on solid-state packing in disordered PC61BM and PC71BM fullerenes. J. Phys. Chem. Lett. 5, 3427–3433 (2014).
Zhang, R., Futagoishi, T., Murata, M., Wakamiya, A. & Murata, Y. Synthesis and structure of an open-cage thiafullerene C69S: reactivity differences of an open-cage C70 tetraketone relative to its C60 analogue. J. Am. Chem. Soc. 136, 8193–8196 (2014).
Krachmalnicoff, A., Levitt, M. H. & Whitby, R. J. An optimized scalable synthesis of H2O@C60 and a new synthesis of H2@C60 . Chem. Commun. 50, 13037–13040 (2014).
Olmstead, M. M. et al. Interaction of curved and flat molecular surfaces. The structure of crystalline compounds composed of fullerene (C60, C60O, C70, and C120O) and metal octaethylporphyrin units. J. Am. Chem. Soc. 121, 7090–7097 (1999).
Frisch, M. J. et al. GAUSSIAN 09 (Gaussian, Inc., 2010).
Chung, L. et al. The ONIOM method and its applications. Chem. Rev. 115, 5678–5796 (2015).
Cheeseman, J. R., Trucks, G. W., Keith, T. A. & Frisch, M. J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 104, 5497–5509 (1996).
Holzapfel, W. & Drickamer, H. G. Effect of pressure on the volume of the high-pressure (VII) phase of H2O and D2O. J. Chem. Phys. 48, 4798–4800 (1968).
Isaacs, E. D. et al. Covalency of the hydrogen bond in ice: a direct X-ray measurement. Phys. Rev. Lett. 82, 600–603 (1999).
Sartori, E. et al. Nuclear relaxation of H2 and H2@C60 in organic solvents. J. Am. Chem. Soc. 128, 14752–14753 (2006).
Li, Y. et al. Comparison of nuclear spin relaxation of H2O@C60 and H2@C60 and their nitroxide derivatives. J. Phys. Chem. Lett. 3, 1165–1168 (2012).
Lang, E. & Lüdemann, H.-D. Pressure and temperature dependence of the longitudinal proton relaxation times in supercooled water to –87 °C and 2500 bar. J. Chem. Phys. 67, 718–723 (1977).
Masutani, K. & Ochiai, S. in Introduction to Experimental Infrared Spectroscopy: Fundamentals and Practical Methods (ed. Tasumi, M.) 141–152 (Wiley, 2015).
Refson, K. & Parker, S. F. Assignment of the internal vibrational modes of C70 by inelastic neutron scattering spectroscopy and periodic DFT. ChemistryOpen 4, 620–625 (2015).
Uhlik, F. et al. Water-dimer stability and its fullerene encapsulations. J. Comp. Theor. Nano. 12, 959–964 (2015).
Kayal, A. & Chandra, A. Exploring the structure and dynamics of nano-confined water molecules using molecular dynamics simulations. Mol. Simul. 41, 463–470 (2015).
Nomura, K. & Okada, S. An anomalous dipole–dipole arrangement of water molecules encapsulated into C60 dimer. Chem. Phys. Lett. 608, 351–354 (2014).
Acknowledgements
Financial support was partially provided by the PRESTO programme on ‘Molecular Technology and Creation of New Functions’ from the JST, the JSPS KAKENHI (grant no. 23241032 and 15K13641) and Grant-in-Aids for Scientific Research on Innovative Areas ‘π-System Figuration: Control of Electron and Structural Dynamism for Innovative Functions’ and ‘Stimuli-responsive Chemical Species for the Creation of Functional Molecules’ from the MEXT, Japan. NMR measurements were supported by the Joint Usage/Research Center (JURC) at the ICR, Kyoto University. The authors thank T. Futagoishi, O. Tomita, H. Kunioku, R. Abe, Y. Kitazumi and K. Kano for support with X-ray and infrared measurements.
Author information
Authors and Affiliations
Contributions
Y.M. conceived and designed the projects. R.Z. carried out most of the experimental works and theoretical calculations, and wrote the paper supported by M.M., T.A. and A.W. T.S. and T.H. performed the infrared measurements.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 11942 kb)
Supplementary information
Crystallographic data for compound H2O@C70. (CIF 61 kb)
Supplementary information
Structure factors file for compound H2O@C70. (FCF 637 kb)
Rights and permissions
About this article
Cite this article
Zhang, R., Murata, M., Aharen, T. et al. Synthesis of a distinct water dimer inside fullerene C70. Nature Chem 8, 435–441 (2016). https://doi.org/10.1038/nchem.2464
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2464
This article is cited by
-
Synthesis of endohedral fullerenes by molecular surgery
Communications Chemistry (2022)
-
C70 Fullerene Cage as a Novel Catalyst for Efficient Proton Transfer Reactions between Small Molecules: A Theoretical study
Scientific Reports (2019)
-
Modification of boron nitride nanocages by titanium doping results unexpectedly in exohedral complexes
Nature Communications (2019)
-
The influence of the configuration of the (C70)2 dimer on its rovibrational spectroscopic properties: a theoretical survey
Journal of Molecular Modeling (2018)
-
Performance of wave function and density functional methods for water hydrogen bond spin–spin coupling constants
Journal of Molecular Modeling (2017)