Abstract
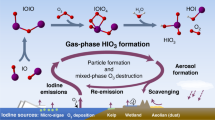
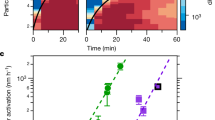
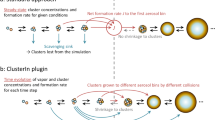
Homogeneous nucleation and subsequent cluster growth leads to the formation of new aerosol particles in the atmosphere1. The nucleation of sulfuric acid and organic vapours is thought to be responsible for the formation of new particles over continents1,2, whereas iodine oxide vapours have been implicated in particle formation over coastal regions3,4,5,6,7. The molecular clustering pathways that are involved in atmospheric particle formation have been elucidated in controlled laboratory studies of chemically simple systems2,8,9,10, but direct molecular-level observations of nucleation in atmospheric field conditions that involve sulfuric acid, organic or iodine oxide vapours have yet to be reported11. Here we present field data from Mace Head, Ireland, and supporting data from northern Greenland and Queen Maud Land, Antarctica, that enable us to identify the molecular steps involved in new particle formation in an iodine-rich, coastal atmospheric environment. We find that the formation and initial growth process is almost exclusively driven by iodine oxoacids and iodine oxide vapours, with average oxygen-to-iodine ratios of 2.4 found in the clusters. On the basis of this high ratio, together with the high concentrations of iodic acid (HIO3) observed, we suggest that cluster formation primarily proceeds by sequential addition of HIO3, followed by intracluster restructuring to I2O5 and recycling of water either in the atmosphere or on dehydration. Our study provides ambient atmospheric molecular-level observations of nucleation, supporting the previously suggested role of iodine-containing species in the formation of new aerosol particles3,4,5,6,7,12,13,14,15,16,17,18, and identifies the key nucleating compound.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kulmala, M. et al. Chemistry of atmospheric nucleation: on the recent advances on precursor characterization and atmospheric cluster composition in connection with atmospheric new particle formation. Annu. Rev. Phys. Chem. 65, 21–37 (2014)
Riccobono, F. et al. Oxidation products of biogenic emissions contribute to nucleation of atmospheric particles. Science 344, 717–721 (2014)
O’Dowd, C. D. et al. Marine aerosol formation from biogenic iodine emissions. Nature 417, 632–636 (2002)
Yoon, Y. J., O’Dowd, C. D., Jennings, S. G. & Lee, S. H. Statistical characteristics and predictability of particle formation events in Mace Head. J. Geophys. Res. 111, D13204 (2006)
O’Dowd, C. D. & de Leeuw, G. Marine aerosol production: a review of the current knowledge. Phil. Trans. R. Soc. A 365, 1753–1774 (2007)
McFiggans, G. et al. Iodine-mediated coastal particle formation: an overview of the Reactive Halogens in the Marine Boundary Layer (RHaMBLe) Roscoff coastal study. Atmos. Chem. Phys. 10, 2975–2999 (2010)
Mahajan, A. S. et al. Concurrent observations of atomic iodine, molecular iodine and ultrafine particles in a coastal environment. Atmos. Chem. Phys. 11, 2545–2555 (2011)
Kirkby, J. et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 476, 429–433 (2011)
Kürten, A. et al. Neutral molecular cluster formation of sulfuric acid-dimethylamine observed in real time under atmospheric conditions. Proc. Natl Acad. Sci. USA 111, 15019–15024 (2014)
Kirkby, J. et al. Ion-induced nucleation of pure biogenic particles. Nature 533, 521–526 (2016)
Kulmala, M. et al. Direct observations of atmospheric aerosol nucleation. Science 339, 943–946 (2013)
Hoffman, T., O’Dowd, C. D. & Seinfeld, J. H. Iodine oxide homogeneous nucleation: an explanation for coastal new particle production. Geophys. Res. Lett. 28, 1949–1952 (2001)
Jimenez, J. L. et al. New particle formation from photooxidation of diiodomethane (CH2I2). J. Geophys. Res. 108, 4318 (2003)
McFiggans, G. et al. Direct evidence for coastal iodine particles from Laminaria macroalgae: linkage to emissions of molecular iodine. Atmos. Chem. Phys. 4, 701–713 (2004)
Saiz-Lopez, A. & Plane, J. M. C. Novel iodine chemistry in the marine boundary layer. Geophys. Res. Lett. 31, L04112 (2004)
Saunders, R. W. & Plane, J. M. C. Formation pathways and composition of iodine oxide ultra-fine particles. Environ. Chem. 2, 299–303 (2005)
Saunders, R. W. et al. Studies of the formation and growth of aerosol from molecular iodine precursor. Z. Phys. Chem. 224, 1095–1117 (2010)
Ehn, M. et al. Growth rates during coastal and marine new particle formation in western Ireland. J. Geophys. Res. 115, D18218 (2010)
Huang, R.-J. et al. In situ measurements of molecular iodine in the marine boundary layer: the link to macroalgae and the implications for O3, IO, OIO and NOx . Atmos. Chem. Phys. 10, 4823–4833 (2010)
Alicke, B., Hebestreit, K., Stutz, J. & Platt, U. Iodine oxide in the marine boundary layer. Nature 397, 572–573 (1999)
Atkinson, R. et al. Evaluated kinetic and photochemical data for atmospheric chemistry. Supplement VIII: halogen species evaluation for atmospheric chemistry. J. Phys. Ref. Data 29, 167–266 (2000)
Bloss, W. J., Rowley, D. M., Cox, R. A. & Jones, R. L. Kinetics and products of the IO self-reaction. J. Phys. Chem. A 105, 7840–7854 (2001)
Sunder, S. & Vikis, A. C. Raman spectra of iodine oxyacids produced by the gas-phase reaction of iodine with ozone in the presence of water vapour. Can. J. Spectros. 32, 45–48 (1987)
O’Dowd, C. D. On the spatial extent and evolution of coastal aerosol plumes. J. Geophys. Res. 107, (2002)
Ehn, M. et al. A large source of low-volatility secondary organic aerosol. Nature 506, 476–479 (2014)
Saiz-Lopez, A. et al. Atmospheric chemistry of iodine. Chem. Rev. 112, 1773–1804 (2012)
Mahajan, A. S. et al. Measurement and modelling of tropospheric reactive halogen species over the tropical Atlantic Ocean. Atmos. Chem. Phys. 10, 4611–4624 (2010)
Lawler, M. J., Mahajan, A. S., Saiz-Lopez, A. & Saltzman, E. S. Observations of I2 at a remote marine site. Atmos. Chem. Phys. 14, 2669–2678 (2014)
Saiz-Lopez, A. et al. On the vertical distribution of boundary layer halogens over coastal Antarctica: implications for O3, HOx, NOx and the Hg lifetime. Atmos. Chem. Phys. 8, 887–900 (2008)
Mahajan, A. S. et al. Evidence of reactive iodine chemistry in the Arctic boundary layer. J. Geophys. Res. 115, D20303 (2010)
Atkinson, H. M. et al. Iodine emissions from the sea ice of the Weddell Sea. Atmos. Chem. Phys. 12, 11229–11244 (2012)
Allan, J. et al. Iodine observed in new particle formation events in the Arctic atmosphere during ACCACIA. Atmos. Chem. Phys. 15, 5599–5609 (2015)
Roscoe, H. K. Particles and iodine compounds in coastal Antarctica. J. Geophys. Res. 120, 7144–7156 (2015)
Acknowledgements
This work was partly funded by the Academy of Finland (Centre of Excellence Project 1118615 and Projects 251427 and 266388), the PEGASOS project (funded by the European Commission under the Framework Program 7 (FP7-ENV-2010-265148)), ACTRIS, the EPA-Ireland, the Nordic Centre of Excellence (CRAICC), the Finnish Antarctic Research Program and the European Research Council (ATMNUCLE grant 227463, MOCAPAF grant 257360 and COALA grant 638703).
Author information
Authors and Affiliations
Contributions
M.S., N.S., T.J., S.R., J.Ka., A.F. and O.P. performed the field measurements. M.S., N.S., T.J., J.Ko. and H.J. analysed the data. H.H. suggested the chemical mechanism and performed quantum chemical calculations. S.R., T.B. and M.S. performed the laboratory experiments. M.S., V.M.K. and C.O. wrote the manuscript. M.S., M.K., H.J. and C.O. designed the measurements. D.C. and C.O. organized the field study at Mace Head and contributed to the data analysis. All authors contributed to data interpretation and to the development of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
TofTools for Matlab used for processing the mass spectrometer data is available upon request from the corresponding author.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Tables 1-2, Supplementary References and Supplementary Figures 1-15. (PDF 8185 kb)
Rights and permissions
About this article
Cite this article
Sipilä, M., Sarnela, N., Jokinen, T. et al. Molecular-scale evidence of aerosol particle formation via sequential addition of HIO3. Nature 537, 532–534 (2016). https://doi.org/10.1038/nature19314
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19314
This article is cited by
-
Natural short-lived halogens exert an indirect cooling effect on climate
Nature (2023)
-
Atmospheric new particle formation from the CERN CLOUD experiment
Nature Geoscience (2023)
-
The gas-phase formation mechanism of iodic acid as an atmospheric aerosol source
Nature Chemistry (2023)
-
Increased aerosol concentrations in the High Arctic attributable to changing atmospheric transport patterns
npj Climate and Atmospheric Science (2022)
-
Climate changes modulated the history of Arctic iodine during the Last Glacial Cycle
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.