Abstract
Major depressive disorder affects around 16 per cent of the world population at some point in their lives. Despite the availability of numerous monoaminergic-based antidepressants, most patients require several weeks, if not months, to respond to these treatments, and many patients never attain sustained remission of their symptoms. The non-competitive, glutamatergic NMDAR (N-methyl-d-aspartate receptor) antagonist (R,S)-ketamine exerts rapid and sustained antidepressant effects after a single dose in patients with depression, but its use is associated with undesirable side effects. Here we show that the metabolism of (R,S)-ketamine to (2S,6S;2R,6R)-hydroxynorketamine (HNK) is essential for its antidepressant effects, and that the (2R,6R)-HNK enantiomer exerts behavioural, electroencephalographic, electrophysiological and cellular antidepressant-related actions in mice. These antidepressant actions are independent of NMDAR inhibition but involve early and sustained activation of AMPARs (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors). We also establish that (2R,6R)-HNK lacks ketamine-related side effects. Our data implicate a novel mechanism underlying the antidepressant properties of (R,S)-ketamine and have relevance for the development of next-generation, rapid-acting antidepressants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
13 May 2016
The competing financial interests statement did not display correctly online when this paper was first published; this has been corrected and the statement is now available.
References
Kessler, R.C. et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289, 3095–3105 (2003)
Trivedi, M. H. et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163, 28–40 (2006)
Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917 (2006)
Zarate, C. A., Jr et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864 (2006)
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000)
Diazgranados, N. et al. A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 67, 793–802 (2010)
Lally, N. et al. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl. Psychiatry 4, e469 (2014)
Murrough, J. W. et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 170, 1134–1142 (2013)
Morgan, C. J. & Curran, H. V. Ketamine use: a review. Addiction 107, 27–38 (2012)
Newport, D. J. et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 172, 950–966 (2015)
Leung, L. Y. & Baillie, T. A. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J. Med. Chem. 29, 2396–2399 (1986)
Moaddel, R. et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur. J. Pharmacol. 698, 228–234 (2013)
Ebert, B., Mikkelsen, S., Thorkildsen, C. & Borgbjerg, F. M. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur. J. Pharmacol. 333, 99–104 (1997)
Yang, C. et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry 5, e632 (2015)
Maeng, S. et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry 63, 349–352 (2008)
Autry, A. E. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95 (2011)
Desta, Z. et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica 42, 1076–1087 (2012)
Adams, J. D., Jr, Baillie, T. A. & Trevor, A. J. & Castagnoli, N. Jr. Studies on the biotransformation of ketamine. 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed. Mass Spectrom. 8, 527–538 (1981)
Zarate, C. A., Jr et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol. Psychiatry 72, 331–338 (2012)
Carrier, N. & Kabbaj, M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34 (2013)
Franceschelli, A., Sens, J., Herchick, S., Thelen, C. & Pitychoutis, P. M. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience 290, 49–60 (2015)
Irifune, M., Shimizu, T., Nomoto, M. & Fukuda, T. Involvement of N-methyl-d-aspartate (NMDA) receptors in noncompetitive NMDA receptor antagonist-induced hyperlocomotion in mice. Pharmacol. Biochem. Behav. 51, 291–296 (1995)
Gant, T. G. Using deuterium in drug discovery: leaving the label in the drug. J. Med. Chem. 57, 3595–3611 (2014)
Duman, R. S., Aghajanian, G. K., Sanacora, G. & Krystal, J. H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nature Med. 22, 238–249 (2016)
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964 (2010)
Koike, H., Iijima, M. & Chaki, S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav. Brain Res. 224, 107–111 (2011)
Koike, H. & Chaki, S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav. Brain Res. 271, 111–115 (2014)
Whittington, M. A., Traub, R. D., Kopell, N., Ermentrout, B. & Buhl, E. H. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int. J. Psychophysiol. 38, 315–336 (2000)
Cunningham, M. O., Davies, C. H., Buhl, E. H., Kopell, N. & Whittington, M. A. Gamma oscillations induced by kainate receptor activation in the entorhinal cortex in vitro. J. Neurosci. 23, 9761–9769 (2003)
Muthukumaraswamy, S. D. et al. Evidence that Subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J. Neurosci. 35, 11694–11706 (2015)
Lepack, A. E., Fuchikami, M., Dwyer, J. M., Banasr, M. & Duman, R. S. BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 18, pyu033 (2015)
De Vry, J. & Jentzsch, K. R. Role of the NMDA receptor NR2B subunit in the discriminative stimulus effects of ketamine. Behav. Pharmacol. 14, 229–235 (2003)
Moaddel, R. et al. The distribution and clearance of (2S,6S)-hydroxynorketamine, an active ketamine metabolite, in Wistar rats. Pharmacol. Res. Perspect. 3, e00157 (2015)
Donahue, R. J., Muschamp, J. W., Russo, S. J., Nestler, E. J. & Carlezon, W. A. Jr. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol. Psychiatry 76, 550–558 (2014)
Malkesman, O. et al. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol. Psychiatry 67, 864–871 (2010)
Zanos, P. et al. The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/glycineb-site inhibition. J. Pharmacol. Exp. Ther. 355, 76–85 (2015)
Chiu, J. et al. Chronic ethanol exposure alters MK-801 binding sites in the cerebral cortex of the near-term fetal guinea pig. Alcohol 17, 215–221 (1999)
Raver, S. M., Haughwout, S. P. & Keller, A. Adolescent cannabinoid exposure permanently suppresses cortical oscillations in adult mice. Neuropsychopharmacology 38, 2338–2347 (2013)
Bokil, H., Andrews, P., Kulkarni, J. E., Mehta, S. & Mitra, P. P. Chronux: a platform for analyzing neural signals. J. Neurosci. Methods 192, 146–151 (2010)
Acknowledgements
We thank A. Keller for his assistance with the qEEG experiments, M. K. Lobo for assistance with the social defeat experiments, B. Alkondon for the rat hippocampal slice preparation and biocytin processing, V. Meadows and S. Krimmel for assistance with behavioural experiments and manuscript review, E. Pereira for critical comments on the manuscript and figures, D. Luckenbaugh for assistance with statistical analysis, and C. Moore for the small molecule X-ray crystallography. Research was supported by NIMH grants MH099345 and MH107615 to T.D.G. and MH086828 to S.M.T., and the NIA (R.M., I.W.W.), NIMH (C.A.Z.), and NCATS (C.J.T.) NIH intramural research programs. Receptor binding profiles and Ki determinations were supported by the NIMH Psychoactive Drug Screening Program, Contract HHSN-271-2008-025C, to B. L. Roth in conjunction with J. Driscoll. Initial synthesis of the ketamine metabolites used in this study was supported by NIA Contract HHSN271201000008I to I.W.W.
Author information
Authors and Affiliations
Contributions
P.Z., R.M., P.J.M., I.W.W., C.J.T., C.A.Z. and T.D.G. were responsible for the overall experimental design. P.J.M., Y.F. and C.J.T. synthesised the ketamine metabolites and deuterated ketamine derivatives, and provided mass spectrometer confirmations. Bioanalytical quantitation of ketamine and metabolites were performed by R.M., N.S.S. and K.S.S.D.. P.Z., P.G. and H.J.P. conducted and analysed the results of the behavioural and qEEG experiments. X.-P.H. supervised and analysed the results of the binding experiments. P.Y. performed the western blot experiments. E.X.A., M.A., J.F. and S.M.T. helped design and analyse the electrophysiology experiments, which were conducted by M.A., J.F. and S.M.T. G.I.E. and C.L.M. conducted and analysed the results of the i.v. self-administration. P.Z. and T.D.G. outlined and wrote the paper, which was reviewed by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing financial interests: I.W.W., R.M., and C.A.Z. are listed as co-inventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. They have assigned their rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. I.W.W., C.A.Z., R.M., T.G., P.Z., C.T., and P.M. are listed as co-inventors on a patent application for the use of (2R, 6R)-hydroxynorketamine and (2S, 6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorders. I.W.W., C.A.Z., R.M., C.T., and P.M. have assigned their rights in this patent to the U.S. government but will share a percentage of any royalties that may be received by the government. T.G. and P.Z. have assigned their rights in this patent to the University of Maryland but will share a percentage of any royalties that may be received by the University of Maryland.
Extended data figures and tables
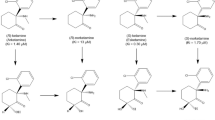
Extended Data Figure 1 The metabolic transformations of ketamine in vivo.
Ketamine is metabolised in vivo via P450 enzymatic transformations. i, (R,S)-KET is selectively demethylated to give (R,S)-norketamine (norKET). ii, NorKET can be then dehydrogenated to give (R,S)-dehydronorketamine (DHNK). iii, Alternatively, norKET can be hydroxylated to give the hydroxynorketamines (HNKs). iv, (R,S)-KET can also be hydroxylated at the 6 position to give either the E-6-hydroxyketamine ((2S,6R;2R,6S)-HK) or Z-6-hydroxyketamine ((2S,6S;2R,6R)-HK). v, Demethylation of (2S,6R;2R,6S)-HK yields the production of (2S,6R;2R,6S)-HNK. vi, Demethylation of (2S,6S;2R,6R)-HK further gives (2S,6S;2R,6R)-HNK.
Extended Data Figure 2 Circulating levels of ketamine and its metabolites following i.p. administration in mice.
a, b, Plasma (a) and brain (b) levels of ketamine and its metabolites after administration of (R,S)-KET (10 mg kg−1) in mice. c, d, Brain levels of KET (c), norKET (d) and HNK (e) following administration of (S)- and (R)-KET. f, g, Chemical structure of (R,S)-6,6-dideuteroketamine ((R,S)-d2-KET) (f), which displaces [3H]MK-801 binding with a similar affinity to (R,S)-KET ((R,S)-KET: Ki = 799 nM; (R,S)-d2-KET: Ki = 883 nM) (g). See Supplementary Table 1 for statistical analyses and n numbers.
Extended Data Figure 3 Additional social defeat stress data.
a, Chronic social defeat stress and social interaction/avoidance test timeline. b, c, 24 h after administration, neither (R,S)-KET nor MK-801 affected locomotor activity (b) or total number of compartmental crosses in the social interaction apparatus (c). Data are mean ± s.e.m. ***P < 0.001. See Supplementary Table 1 for statistical analyses and n numbers.
Extended Data Figure 4 Locomotor effects of (R,S)-KET and (R,S)-d2-KET.
After recording baseline activity for 60 min, mice received drug (marked by a vertical dashed line) and locomotor activity was monitored for another 1 h. a, b, Administration of (R,S)-KET (10 mg kg−1), induced hyperlocomotor responses equally in both male and female mice. c, d, (R,S)-KET and (R,S)-d2-KET were equally potent in inducing a hyperlocomotor response at the dose of 10 mg kg−1. Data are mean ± s.e.m. *P < 0.05, **P < 0.01 (see Supplementary Table 1 for statistical analyses and n numbers).
Extended Data Figure 5 Acute and sustained antidepressant and anti-anhedonic effects of (2R,6R)- and (2S,6S)-HNK.
a, A single injection of (2R,6R)-HNK resulted in dose-dependent antidepressant-like responses in the learned helplessness test at the doses of 5–75 mg kg−1. b, A single injection of (2S,6S)- HNK induced antidepressant-like effects in the learned helplessness test at the dose of 75 mg kg−1. c, Administration of (2R,6R)-HNK induced dose-dependent antidepressant effects in the 1- and 24-h FST. d, Administration of (2S,6S)-HNK at the dose of 25 mg kg−1 induced antidepressant effects in the 1- and 24-h FST. e, Despite the greater antidepressant efficacy of (2R,6R)-HNK, administration of (2S,6S)-HNK (HNK) results in higher brain hydroxynorketamine levels compared to (2R,6R)-HNK. f, (2R,6R)-HNK manifested dose-dependent antidepressant-like effects in the NSF test. g, Similar to (R,S)-KET, the antidepressant-like effects of (2R,6R)-HNK in the FST persisted for at least 3 days after treatment. h, A single administration of (2R,6R)-HNK reversed chronic corticosterone-induced decreases in sucrose preference. i, A single administration of (2R,6R)-HNK reversed chronic corticosterone-induced decrease in female urine sniffing preference, specifically in mice that developed an anhedonic phenotype. Administration of (2R,6R)-HNK was not associated with changes in locomotor activity (j) or total compartmental crosses in the social interaction test after chronic social defeat stress (k). Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 (see Supplementary Table 1 for statistical analyses and n numbers).
Extended Data Figure 6 (2R,6R)-HNK rapidly increases the frequency and amplitude of AMPAR spontaneous excitatory postsynaptic currents in the hippocampus.
a, Representative traces of spontaneous excitatory postsynaptic currents (sEPSCs) mediated via AMPARs during baseline (before) and 20 min after drug administration. b, Example CA1 stratum radiatum interneuron recorded from a rat hippocampal slice. c–j, Twenty-minute exposure of (2R,6R)-HNK (e, i), but not (R,S)-(KET) (c, g) or (2S,6S)-HNK (d, h), increased AMPA sEPSCs frequency and amplitude compared to baseline. Data are mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 (see Supplementary Table 1 for statistical analyses and n numbers). SLM, stratum lacunosum-moleculare; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum.
Extended Data Figure 7 Administration of the AMPAR antagonist, NBQX, prevents (2R,6R)-HNK-induced increases in gamma oscillations in vivo.
a, Administration of (R,S)-KET, but not (2R,6R)- HNK, increased locomotor home-cage activity of mice. b–e, Neither (R,S)-KET nor (2R,6R)-HNK altered cortical alpha (b), beta (c), delta (d) or theta (e) oscillations in vivo. f–k, Pre-treatment with the AMPAR antagonist, NBQX, did not change the locomotor activity (f), alpha (g), beta (h), delta (j) or theta (k) oscillations, but it prevented (2R,6R)-HNK-induced increases of gamma oscillations in vivo (i). Data are mean ± s.e.m. *P < 0.05, **P < 0.01 (see Supplementary Table 1 for statistical analyses and n numbers).
Extended Data Figure 8 Effects of (2R,6R)-hydroxynorketamine on protein and protein phosphorylation levels in synaptoneurosomes.
a, b, A single administration of (R,S)-KET (10 mg kg−1) or (2R,6R)- HNK (10 mg kg−1), did not alter levels of mTOR or phosphorylated mTOR in the hippocampus 1 h (a) or 24 h (b) after injection. c–j, Administration of (R,S)-KET or (2R,6R)-HNK did not alter levels of mTOR/phosphorylated mTOR (c, d), eEF2/phosphorylated eEF2 (e, f), proBDNF/mBDNF (g, h), or GluA1/GluA2 (i, j), in the prefrontal cortex of mice. The values for the phosphorylated forms of proteins were normalized to phosphorylation-independent levels of the same protein. Phosphorylation-independent levels of proteins were normalized to GAPDH. Data are mean ± s.e.m, and were normalized to the saline-treated control group for each protein. Images are cropped; see Supplementary Fig. 1 for complete blot images. *P < 0.05 (see Supplementary Table 1 for statistical analyses and n numbers).
Extended Data Figure 9 (2R,6R)-HNK administration does not alter startle amplitude, drug discrimination rate or self-administration drug intake.
a, Startle amplitude as measured in the pre-pulse inhibition task was not affected by administration of (R,S)-KET or (2R,6R)-HNK. b, c, Response rate of overall lever pressing per sec in the drug discrimination model was not changed by administration of (R,S)-KET, (2R,6R)-HNK (b) or PCP (c). d, Unlike ketamine, (2R,6R)-HNK did not alter drug intake in the self-administration task in mice. Data are mean ± s.e.m. *P < 0.05 (see Supplementary Table 1 for statistical analyses and n numbers).
Supplementary information
Supplementary Information
This file contains (1) Supplementary Table 1, which includes the details for the number of animals and the results of the statistical analyses in each experiment; (2) Supplementary Figure 1, with the complete blot images for Figure 4 and Extended Data Figure 8; (3) the Supplementary Methods for the synthesis of (2R,6R)-HNK, (2S,6S)-HNK, and D-Ket, in addition to the analytical data that supports the synthetic steps and the X-ray crystallographic data that confirms the absolute and relative conformation for (2S,6S)-HNK and (2R,6R)-HNK. (PDF 7157 kb)
Source data
Rights and permissions
About this article
Cite this article
Zanos, P., Moaddel, R., Morris, P. et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486 (2016). https://doi.org/10.1038/nature17998
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17998
This article is cited by
-
Targeting metaplasticity mechanisms to promote sustained antidepressant actions
Molecular Psychiatry (2024)
-
The role of neurotrophic factors in novel, rapid psychiatric treatments
Neuropsychopharmacology (2024)
-
Ketamine in neuropsychiatric disorders: an update
Neuropsychopharmacology (2024)
-
Sex dependence of opioid-mediated responses to subanesthetic ketamine in rats
Nature Communications (2024)
-
EEG-vigilance regulation is associated with and predicts ketamine response in major depressive disorder
Translational Psychiatry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.