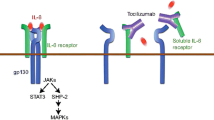
Abstract
Sarilumab (Kevzara™) is a fully human IgG1 monoclonal antibody that binds specifically to both soluble and membrane-bound interleukin (IL)-6 receptors (sIL-6Rα and mIL-6Rα) and thereby inhibits IL-6-mediated signalling through these receptors. Subcutaneous sarilumab is approved in Canada for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more biological or non-biological disease-modifying anti-rheumatic drugs. It is under regulatory review for use in rheumatoid arthritis in other countries, including in the EU, USA and Japan. Sarilumab is also under phase II investigation for the treatment of juvenile idiopathic arthritis. This article summarizes the milestones in the development of sarilumab leading to its first global approval for the treatment of rheumatoid arthritis.
Similar content being viewed by others
References
Sanofi-aventis Canada Inc. Kevzara™ (sarilumab): product monograph (Canada). 2017. http://pdf.hres.ca/dpd_pm/00037766.PDF. Accessed 6 Mar 2017.
Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatois arthritis. Ther Adv Musculoskel Dis. 2010;2(5):247–56.
Smolen JS, Aletaha D, McInnes IA. Rheumatoid arthritis. Lancet. 2016;388:23–38.
Sanofi, Regeneron Pharmaceuticals. Sanofi and Regeneron announce EMA acceptance for review of Marketing Authorisation Application for sarilumab. 2016. http://www.sanofi.com. Accessed 15 Feb 2017.
Sanofi, Regeneron Pharmaceuticals Inc. Sanofi and Regeneron announce first approval of KevzaraTM (sarilumab) for the treatment of moderately to severely active rheumatoid arthritis in adult patients by Health Canada. 2017. http://www.sanofi-aventis.com. Accessed 1 Feb 2017.
Regeneron Pharmaceuticals. Regeneron report third quarter 2016 financial and operating results. Media release. 2016. http://newsroom.regeneron.com/. Accessed 1 Feb 2017.
Sanofi, Regeneron Pharmaceuticals. Juvenile idiopathic arthritis: NCT02776735 and NCT02991469. 2017. http://clinicaltrials.gov. Accessed 15 Feb 2017.
Sanofi, Regeneron Pharmaceuticals. Ankylosing spondylitis: NCT01118728 and NCT01061723. 2017. http://clinicaltrials.gov/. Accessed 15 Feb 2017.
Mangan EK, Parrino J, Wu R, et al. Pharmacodynamic effect and safety of single-dose administration of subcutaneous sarilumab or intravenous tocilizumab in patients with rheumatoid arthritis [abstract no. APL16-0768]. Int J Rheum Dis. 2016;19(Suppl 2):196.
Boyapati A, Msihid J, Fiore S, et al. Sarilumab plus methotrexate suppresses circulating biomarkers of bone resorption and synovial damage in patients with rheumatoid arthritis and inadequate response to methotrexate: a biomarker study of MOBILITY. Arthritis Res Ther. 2016;18(1):225.
Huizinga TW, Fleischmann RM, Jasson M, et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. 2014;73:1626–34.
Genovese MC, Fleischmann R, Kivitz AJ, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol. 2015;67(6):1424–37.
Strand V, Kosinski M, Chen CI, et al. Sarilumab plus methotrexate improves patient-reported outcomes in patients with active rheumatoid arthritis and inadequate responses to methotrexate: results of a phase III trial. Arthritis Res Ther. 2016;18:198.
van der Heijde D, Fan C, van Hoogstraten H, et al. Consistency of radiographic responses with sarilumab plus methotrexate across subpopulations of patients with rheumatoid arthritis in a phase 3 study [abstract no. SAT0058]. Ann Rheum Dis. 2016;75(Suppl 2):685.
Fleischmann R, Mangan EK, van Adelsberg J, et al. Onset of action of sarilumab in patients with rheumatoid arthritis in 2 phase 3 studies [abstract no. SAT0180]. Ann Rheum Dis. 2016;75(Suppl 2):733.
Strand V, Chen C, Mahajan P, et al. Early onset of benefit by patient-reported outcomes (PROs) with sarilumab treatment in RA [abstract no. AB0251]. Ann Rheum Dis. 2016;75(Suppl 2):984–5.
Fleischmann R, van Adelsberg J, Lin Y, et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active RA and inadequate response or intolerance to tumour necrosis factor inhibitors. Arthritis Rheumatol. 2016;69(2):277–90.
Strand V, Mahajan P, Chen C, et al. Benefit of sarilumab with csDMARDs on patient productivity in work, household work and family, social, leisure activities in TNF-IR RA patients [abstract no. AB0252]. Ann Rheum Dis. 2016;75(Suppl 2):985.
Burmester GR, Lin Y, Patel R, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2016. doi:10.1136/annrheumdis-2016-210310.
Genovese MC, Fay J, Parrino J, et al. Sarilumab dose reduction to manage laboratory abnormalities in an open-label extension study in RA patients [abstract no. FRI0228]. Ann Rheum Dis. 2016;75(Suppl 2):515–6.
Fleischmann R, Genovese MC, van Adelsberg J, et al. Pooled safety and efficacy of sarilumab in rheumatoid arthritis patients 65 years of age and older [abstract no. 1605]. Arthritis Rheumatol. 2016;68(Suppl 10).
Sanofi, Regeneron Pharmaceuticals. Non-infectious uveitis: NCT01900431. 2017. http://clinicaltrials.gov/. Accessed 15 Feb 2017.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. L.J. Scott is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Scott, L.J. Sarilumab: First Global Approval. Drugs 77, 705–712 (2017). https://doi.org/10.1007/s40265-017-0724-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0724-2