Abstract
In the present investigation, we have synthesized different morphologies of carbon nanofibres (CNFs) to investigate their catalytic effect on the hydrogenation characteristics of 25 h ball-milled MgH2 (nano MgH2). The TEM analysis reveals that 25 h of ball-milling leads to the formation of nanocrystalline particles with size ranging between 10 and 20 nm. Different morphologies of CNFs were synthesized by catalytic thermal decomposition of acetylene (C2H2) gas over LaNi5 alloy. Helical carbon nanofibers (HCNFs) were formed at a temperature 650 °C. By increasing the synthesis temperature to 750 °C, planar carbon nanofibres were formed. In order to explore the effectiveness of CNFs towards lowering the decomposition temperature, TPD experiments (at heating rate 5 °C/min) were performed for nano MgH2 with and without CNFs. It was found that the decomposition temperature is reduced to ~334 and ~300 °C from 367 °C for the PCNF and HCNF catalysed nano MgH2. It is also found that HCNF admixed nano MgH2 absorbs ~5.25 wt% within 10 min as compared with pristine nano MgH2, which absorbs only ~4.2 % within the same time and same condition of temperature and pressure. Thus the HCNF possesses better catalytic activity than PCNF. These different levels of improvement in hydrogenation properties of HCNF catalysed nano MgH2 is attributed to the morphology of the CNFs.
Similar content being viewed by others
Introduction
MgH2 is a potential hydrogen storage material. It satisfies most of the requisites for a viable hydrogen storage system for mobile as well as stationary hydrogen devices. The slow hydriding and dehydriding are the major drawback of the magnesium hydride as hydrogen storage material. Bérubé et al. 2007, reported that the absorption at 600 K is initiated through the hydride formation at the Mg grain surface and that its rate of formation is controlled by the density of nucleation sites. The reaction would then stop when a hydride layer covers the surface of the magnesium preventing fast diffusion to the core of the particle and hence slowing down the kinetics of the β phase transition. Therefore, nano Mg may form a better material version for practical applications (Sakintuna and Lamari-Darkrim 2007). This leads to increase of surface area, creation of defects, and nucleation sites and also reduces the hydrogen diffusion path.
In order to further improve the sorption behaviour of MgH2, several studies have also reported that combination of ball-milling with a catalyst provides significant improvement in hydrogenation characteristics (Jain et al. 2010; Agarwal et al. 2009; Barkhordarian et al. 2006; Hanada et al. 2005; Charbonnier et al. 2004; Bobet et al. 2003; Zaluska et al. 2001; Tessier and Akiba 2000). The main drawback of these additives is the density of the metal and alloys which create a negative weight for storage applications. In recent years carbon nanostructures are being extensively used as a catalyst for improvement of the sorption behaviour of hydrogen storage materials (Wua and Cheng 2010).
Some recent reports have described MgH2 admixed (catalysed) various carbon nanostructures (Imamura et al. 2003; Chen et al. 2004; Wu et al. 2005, 2006; Lillo-Ro’denas et al. 2008; Wu et al. 2010). These studies bring out the beneficial effect of carbon materials on both the hydriding and dehydriding behaviours. Among these results Wu et al. 2010 reported that 5 wt% SWCNTs catalysed MgH2 absorbs 6 and 4.2 wt% of hydrogen at 423 and 373 K, respectively. However, Lillo-Ro’denas et al. 2008 investigated the effect of different carbon materials on decomposition of MgH2. The best results were achieved in the mixtures involving CNFs with metallic impurities (Ni and Fe). Wu et al. (2010) also reported that as prepared nickel-coated carbon nanofibres (NiCNF) exhibit a superior catalytic effect on hydrogenation behaviour of magnesium.
In the present investigation we have synthesized different morphologies of CNFs and investigated their catalytic effect on the hydrogenation behaviour of nano MgH2. Different morphologies of CNFs were synthesized by catalytic thermal decomposition of acetylene (C2H2) gas over LaNi5 alloy at different temperature. The HCNFs were formed at a temperature 650 °C, whereas PCNFs were synthesized at temperature 750 °C.
Experimental details
The samples with and without the catalyst were characterized using powder X-ray diffraction (XRD) (X’ Pert PRO, PANalytical) and transmission electron microscopy (TEM) (FEI, TECNAI 20G2). The XRD specimen holder was covered by a fine layer of parafilm (Pechiney plastic packing) for protecting the sample from air/moisture induced contamination. The hydrogen sorption behaviours of materials were analysed using the computerized Pressure-Concentration-Temperature (PCT) apparatus (AMC-USA).
Nanocrystalline MgH2 (nano MgH2) was synthesized through the ball milling of as received MgH2 (particle size is 50–100 μm as shown in SEM micrograph Fig. 1a) using Ritsh PM-400 ball miller. The milling was done under hydrogen (99.99 %) pressure of 5 atm for durations ranging from 5 to 35 h. It was found that the duration of 25 h is optimum for obtaining nano MgH2. Figure 1b is a representative SEM micrograph of 25 h ball-milled MgH2 revealing the presence of particle size in the range of 500–1,000 nm (0.5–1 μm). It may be pointed out that the SEM analysis reveals the agglomeration of particles. The actual MgH2 particles size as brought out by TEM ranges from 10 to 20 nm (as shown later in Fig. 7). It has been found that ball-milling under hydrogen pressure reduces the content of MgO. Ball-milling under hydrogen atmosphere reduces the oxide content due to the accelerated hydride formation over the surface of free Mg formed during ball milling (Bobet et al. 2003).
CNFs were synthesized by catalytic thermal decomposition of acetylene (C2H2) gas over LaNi5 alloy. It has been optimized that the 100 mg of the catalyst is sufficient for each run in our experimental conditions. The details of the synthesis process have already been described by us (Raghubanshi et al. 2011). Helical carbon nanofibres (HCNFs) were formed at a temperature 650 °C. Figure 2a represents typical TEM micrograph of as synthesized HCNF. This shows that the as grown CNFs have helical morphology and their typical diameter is 150 nm (shown in Fig. 2a). By increasing the synthesis temperature to 750 °C, planar carbon nanofibres (diameter 200 nm) were formed as shown in Fig. 2b. In both the cases the length of CNFs was 6–8 μm.
Admixing of CNFs were performed inside the glove box using locally fabricated mixer (at 4000 rpm) having three chromium nickel stainless steel balls (2 balls of weight 8.5 g, and 1 ball of weight 0.25 g) and vial having a volume of 150 cc (Shahi et al. 2012). This is beneficial since it is known that morphology of carbon nanostructure (CNS) is important in regard to their catalytic activity; the aforementioned method of admixing maintains the structural morphology of admixed CNS, which acts as a catalyst for hydrogenation/dehydrogenation of nano MgH2. By varying the concentration of CNF being admixed in nano MgH2 it is found that the optimum concentration of CNF is 2 wt%.
Results and discussion
Hydrogenation dehydrogenation characteristics of nano MgH2 with and without CNF
In order to investigate the effect of ball-milling and the catalytic effect of CNF, the temperature programmed desorption (TPD) experiments were performed on the nano MgH2 with and without CNF. Figure 3 represents the decomposition profile at a heating rate of 5 °C/min for as received MgH2, nano MgH2, PCNF and HCNF admixed nano MgH2.TPD curves reveal that the decomposition temperature of as received MgH2 is reduced appreciably with ball-milling for 25 h. It illustrates that the as received MgH2 has a sharp decomposition at temperature 422 °C. The decomposition temperature is reduced to 367 °C for ball-milled MgH2. Thus ball-milling reduces the decomposition temperature up to ~55 °C. The decrease in temperature has been outlined by the arrow in Fig. 3. As can be seen from figure the decomposition temperature is reduced to ~334 and ~300 °C for the PCNF and HCNF admixed nano MgH2. Thus the decomposition temperature of nano MgH2 is reduced to ~300 from ~367 °C with admixing of HCNF. It can thus be said that the TPD experiment reveals that the HCNF catalysed nano MgH2 has ~67 °C lower decomposition temperature as compared with pristine nano MgH2. It may be pointed out that the effect of ball milling itself reduces the desorption temperature from 422 to 367 °C. A further decrease of 67 °C takes place due to catalytic effect of HCNF. Thus in comparison with the as received MgH2 there is a total decrease of 122 °C in desorption temperature.
In order to further investigate the catalytic activity of CNF, the isothermal absorption kinetics measurements were also performed for the nano MgH2 with and without CNF. The absorption kinetics plots for nano MgH2 with and without CNF at 300 °C and 2 MPa of hydrogen pressure are shown in Fig. 4. The hydrogen uptake capacity of nano MgH2 is found to be 4.8 wt%. This gets improved with admixing of PCNFs and HCNFs. It is found that the nano MgH2 admixed with PCNF and HCNF absorbs 5.7 and 5.8 wt% of hydrogen, respectively, within 1 h. It is also found that HCNF admixed nano MgH2 absorbs ~5.25 wt% within 10 min as compared with pristine nano MgH2, which absorbs only ~4.2 % within the same time and same condition of temperature and pressure. Thus absorption kinetics of the pristine nano MgH2 improves significantly with admixing of HCNF.
Evaluation of activation energy for absorption of nano MgH2 with and without HCNFs
As a further step towards understanding rehydrogenation, the activation energy Ea for hydrogen absorption of nano MgH2 as well as the HCNF catalysed nano MgH2 has been calculated. The activation energy for hydrogen absorption was estimated from Arrhenius plots. This is calculated from the following equation:
where k is the rate constant, R is gas constant, T is temperature at which absorption experiment is performed.
The absorption curve for MgH2 is not linear; therefore the rate constant k was obtained using the John-Mehl-Avrani model (Avrami 1941). This proposed kinetic model for nucleation and growth in solids is outlined by the following equation:
where α is the extent of the reaction which can be identified with a normalized hydrogen wt% (range from 0 to 1), t is time and for a fixed temperature k and n are constant.
Figure 5a represents the plot of ln[−ln(1−α)] versus ln(t) for different temperatures; the slope of these plots gives lnk. Further the slope of the Arrhenius plot (lnk vs. 1/T) Fig. 5b gives activation energy. The Ea is found to be 92 kJ/mol for nano MgH2. However, for the as received MgH2, the activation energy was found to be 98 kJ/mol [as described in our previous report (Singh et al. 2010)]. Similar method is adopted for the evaluation of activation energy for HCNF admixed nano MgH2. Figure 5c and d describes the results of evaluation of HCNF catalysed nano MgH2; the activation energy is found to be 59.7 kJ/mol.
Structural and microstructural characterization of CNF catalysed nano MgH2
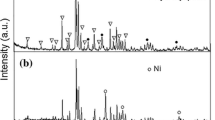
To make these changes explicable, the structure and morphology of the HCNF admixed nano MgH2 is examined by employing XRD and TEM. Figure 6 brings out representative X-ray diffraction patterns of the (a) as synthesized nano MgH2, (b) as synthesized HCNF, (c) HCNF catalysed nano MgH2 and (d) rehydrogenated HCNF catalysed nano MgH2. As can be seen from Fig. 6a, the XRD peaks exhibit considerable broadening. The crystallite size for the nano MgH2 is found to be 10 nm calculated through the Scherrer formula. TEM micrograph (Shown in Fig. 7) also reveals a similar crystallite size distribution. The formation of nano MgH2 through the ball milling is thus evident. The typical XRD pattern of the as synthesized HCNF (Fig. 6b) shows typical graphite XRD peaks (indexed in the figure) together with the synthesis acquired metallic Ni particle. Fig. 6c brings out a representative X-ray diffraction pattern of HCNF admixed nano MgH2; it can be noticed that the peaks corresponding to MgH2 alone are present. Since the catalyst is in very small concentration, it will not be discernible in the XRD pattern.
In order to further understand the morphological changes with admixing of HCNF in nano MgH2, transmission electron microscope studies of the as synthesized nano MgH2 and HCNF catalysed nano MgH2 (which showed the optimum hydrogenation behaviour) are carried out. Figure 7a and b exhibit typical TEM micrograph of the as synthesized (25 h ball milled) nano MgH2. It is found that the synthesized nano MgH2 has particles in the range of 10–20 nm as evident from TEM micrograph. This feature is more clearly visible in the dark field micrograph of synthesized nano MgH2 shown in Fig. 7c. The corresponding electron diffraction pattern obtained from the nano MgH2 is shown in Fig. 7d. The spotty rings were indexed based on the tetragonal structure of βMgH2.
Figure 8a, on the other hand, exhibits representative TEM micrograph of HCNF catalysed nano MgH2. From the microstructure, the presence of CNF can be verified. The microstructure also reveals that HCNFs are dispersed nearly homogeneously in nano MgH2. The selected area electron diffraction pattern representing the microstructure is shown in Fig. 8b. The presence of diagonally opposite arced diffraction spots is signatures of HCNF admixed in nano MgH2 other signs are similar to those shown in Fig. 7d are representing nano MgH2.
The XRD pattern of synthesized CNF revealed that the CNF contained Ni-metallic nano particles as impurity. Thus the catalytic activity of these CNFs may also arise due to the presence of synthesis acquired metal nano particles. In order to understand the influence of Ni-nanoparticles on the catalytic activities of CNFs, we have further investigated the dehydrogenation characteristics of nano MgH2 catalysed separately with Ni-nano particles and purified CNFs. Purification (acid treatment) of as synthesized CNF has been performed with the concentrated HNO3 and washed with distilled water several times. After the acid treatment, it has been found that the morphology of the CNF has not changed; only the concentration of metallic particle becomes negligible (Raghubanshi et al. 2011). From the TPD experiments it has been found that dehydrogenation temperatures for nano MgH2 catalysed separately with purified CNF and Ni-nano particles are 350 and 360 °C, respectively (not shown here), whereas for the case of pristine nano MgH2, the dehydrogenation temperature is found to be 367 °C. Thus the admixing of purified CNF and Ni-nano particle shows only marginal change in dehydrogenation temperature. However, the dehydrogenation temperature of as synthesized PCNF catalysed nano MgH2 is found to be 334 °C. Hence for the present case the combined effect (embedded Ni-nano particles and fibrous morphologies of CNF) is responsible for the catalytic effect of the as synthesized CNFs. Moreover, HCNF possess better catalytic activity as compared with PCNFs since both the CNFs (PCNF and HCNF) have similar synthesised metal nanoparticles (Ni). Hence these different levels of improvement in hydrogenation behaviour of PCNF and HCNF catalysed nano MgH2 may arise due the morphological differences of these two CNFs. Berseth et al. (2009) have also reported that the electronegativity of carbon nanostructures increases with increasing the curvature of carbon nanostructures. Among these CNFs (PCNF and HCNF), the curvature of the HCNFs may increase the electronegativity of HCNFs as compared with PCNFs. Hence for the case of HCNF catalysed nano MgH2, the bonding electrons present in MgH2 may become more delocalized as compared with PCNF catalysed nano MgH2 due to the electronegativity difference between these two CNFs.
Conclusions
The de/re-hydrogenation characteristics of nano MgH2 is improved significantly with admixing of carbon nanofibres. The lowering of decomposition temperature for CNFs catalysed samples as compared with pristine nano MgH2 is from ~367 to ~334 °C and ~300 °C for PCNF and HCNF catalysed nano MgH2, respectively. Thus HCNF possesses better catalytic activity as compared with PCNF. It is also found that the activation energy for rehydrogenation of HCNF catalysed nano MgH2 is ~59.7 kJ/mol, whereas the activation energy for rehydrogenation of pristine nano MgH2 is ~92 kJ/mol. These different levels of improvement in hydrogenation properties of CNF catalysed nano MgH2 is attributed to the morphological differences of these CNFs.
References
Agarwal S, Aurora A, Jain A, Jain IP (2009) Montone A catalytic effect of ZrCrNi alloy on hydriding properties of MgH2. Int J Hydrogen Energy 34:9157–9162
Avrami M (1941) Kinetics of phase change granulation, phase change and microstructures. J Chem Phys 9:177
Barkhordarian G, Klassen T, Bormann R (2006) Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J Alloys Compd 407:249–255
Berseth PA, Harter AG, Zidan R, Blomqvist A, Araujo CM, Scheicher RH et al (2009) Carbon nanomaterials as catalysts for hydrogen uptake and release in NaAlH4. Nano Lett 9:1501–1505
Bérubé B, Radtke G, Dresselhaus M, Chen G (2007) Size effects on the hydrogen storage properties of nanostructured metal hydrides: a review. Int J Hydrogen Energy Res 31:637–663
Bobet JL, Desmoulins-Krawiec S, Grigorova E, Cansell F, Chevalier B (2003) Addition of nanosized Cr2O3 to magnesium for improvement of the hydrogen sorption properties. J Alloys Compd 351:217–221
Charbonnier J, de Rango P, Fruchart D, Miraglia S, Pontonnier L, Rivoirard S, Skryabina N, Vulliet P (2004) Hydrogenation of transition element additives (Ti, V) during ball milling of magnesium hydride. J Alloys Compd 383:205–208
Chen D, Chen L, Liu S, Ma CX, Chen DM, Wang LB (2004) Microstructure and hydrogen storage property of Mg/MWNTs composites. J Alloys Compd 372:231–237
Hanada N, Ichikawa T, Fujii H (2005) Catalytic effect of nanoparticle 3d-transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J Phys Chem B 109:7188–7194
Imamura H, Kusuhara M, Minami S, Matsumoto M, Masanari K, Sakata Y, Itoh K, Fukunaga T (2003) Carbon nanocomposites synthesized by high-energy mechanical milling of graphite and magnesium for hydrogen storage. Acta Mat 51:6407–6414
Jain IP, Lal C, Jain A (2010) Hydrogen storage in Mg: a most promising material. Int J Hydrogen Energy 35:5133–5144
Lillo-Ro’denas MA, Aguey-Zinsou KF, Cazorla-Amoro’s D, Linares-Solano A, Guo ZX (2008) Effect of different carbon materials on MgH2 decomposition. Carbon 46:126–137
Raghubanshi H, Hudson MSL, Srivastava ON (2011) Synthesis of helical carbon nanofibres and its application in hydrogen desorption. Int J Hydrogen Energy 36:4482–4490
Sakintuna B, Lamari-Darkrim F, Hirscher M (2007) Metal hydride materials for solid hydrogen storage: a review. Int J Hydrogen Energy 32:1121–1140
Shahi RR, Raghubanshi H, Shaz MA, Srivastava ON (2012) Studies on the de/rehydrogenation characteristic of Mg(NH2)2/LiH mixture admixed with carbon nanofibres. Int J Hydrogen Energy 37:3705–3711
Singh RK, Raghubanshi H, Pandey SK, Srivastava ON (2010) Effect of mixing different carbon structural variants on the decomposition and hydrogen sorption kinetics of magnesium hydride. Int J Hydrogen Energy 35:4131–4137
Tessier P, Akiba E (2000) Decomposition of nickel-doped magnesium hydride prepared by reactive mechanical alloying. J Alloys Compd 30:215–217
Wu C, Wang P, Yao X, Liu C, Chen D, Lu GQ, Cheng H (2005) Effects of SWNT and metallic catalyst on hydrogen absorption/desorption performance of MgH2. J Phys Chem B 109:22217–22221
Wu C, Wang P, Yao X, Liu C, Chen D, Lu GQ, Cheng H (2006) Effect of carbon/noncarbon addition on hydrogen storage behaviours of Magnesium hydride. J Alloys Compd 414:256–2264
Wu CZ, Yao XD, Zhang H (2010) Hydriding/dehydriding properties of MgH2/5 wt% Ni coated CNFs composite. Int J Hydrogen Energy 35:247–252
Wua C, Cheng HM (2010) Effects of carbon on hydrogen storage performances of hydrides. J Mater Chem 20:5390–5400
Zaluska A, Zalusk L, Ström-Olsen JO (2001) Structure, catalysis and atomic reactions on the nano-scale: a systematic approach to metal hydrides for hydrogen storage. App Phys A: Mat Sci Proc 72:157–165
Acknowledgments
The authors would like to acknowledge Prof. R.S. Tiwari for his encouragement and helpful discussion. Financial assistance received from Ministry of New and Renewable Energy (MNRE), DST (UNANST), UGC-New Delhi (India) and Council of Scientific and Industrial Research (CSIR) is gratefully acknowledged. One of the authors (Mr. Rohit R. Shahi) acknowledges Dr. Kalpana Awasthi for her helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shahi, R.R., Raghubanshi, H., Shaz, M.A. et al. Studies on the de/re-hydrogenation characteristics of nanocrystalline MgH2 admixed with carbon nanofibres. Appl Nanosci 2, 195–201 (2012). https://doi.org/10.1007/s13204-012-0083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0083-y