Abstract
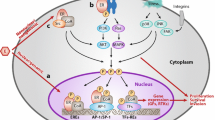
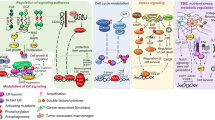
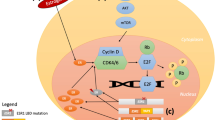
About 70% of breast cancers are hormone receptor (HR) positive, meaning an estrogen receptor (ER) and/or a progesterone receptor (PgR) are present in the tumor. Compounds that modulate ER or PgR signaling, by either competing for estrogen binding to estrogen receptors (synthetic estrogen receptor modulators [eg, tamoxifen]) or opposing the production of estrogen in the body (aromatase inhibitors in postmenopausal women), are dominant among those used to treat HR-positive breast cancer. Although HR-positive tumors are associated with a better prognosis, in the case of advanced disease, most will develop resistance to hormonal treatments over time. Multiple mechanisms of endocrine resistance have been proposed, including ER receptor loss and cross-talk between the ER and HER2/neu (human epidermal growth factor receptor 2; also known as ErbB-2) receptor signaling pathways. The phosphoinositide 3-kinase (PI3K)/phosphatase and tensin homologue deleted on chromosome ten (PTEN)/Akt/mammalian target of rapamycin (mTOR) pathway is often implicated in sensitivity and resistance to therapy. Dysregulated signaling through the PI3K/PTEN/Akt/mTOR pathway may result from genetic alterations in critical components in this pathway. Therapeutic targeting of alternate pathways of growth signaling in ER-positive tumors has the potential to translate into practice-changing therapeutic strategies. This article discusses the concept of primary and secondary endocrine resistance, outlines the proposed mechanisms, and reviews the emerging evidence for interventions designed to overcome resistance and restore ER sensitivity. The recently reported result of the BOLERO-2 study using an mTOR inhibitor (everolimus or RAD001) in combination with an aromatase inhibitor (exemestane) is discussed in some detail.
Similar content being viewed by others
References
Papers of particular interest, published recently have been highlighted as: • Of importance •• Of major importance
Nassa G, Tarallo R, Guzzi PH, Ferraro L, Cirillo F, Ravo M et al. Comparative analysis of nuclear estrogen receptor alpha and beta interactomes in breast cancer cells. Mol BioSyst. 2010.
Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, et al. Estrogen receptor pathways to AP-1. The Journal of steroid biochemistry and molecular biology. 2000;74(5):311–7.
Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocrine Relat Canc. 2004;11(4):643.
Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21(10):1973.
Creighton CJ, Massarweh S, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, et al. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res. 2008;68(18):7493.
Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47.
Khasraw M, Brogi E, Seidman AD. The need to examine metastatic tissue at the time of progression of breast cancer: is re-biopsy a necessity or a luxury? Curr Oncol Rep. 2011;13(1):17–25.
Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134(7):48–72.
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087.
Nilsson S, Gustafsson J-Ö. Estrogen Receptors: Their Actions and Functional Roles in Health and Disease; Nuclear Receptors. In: Bunce CM, Campbell MJ, editors. Proteins and cell regulation, vol. 8. Netherlands: Springer; 2010. p. 91–141.
Palmieri C, Cheng G, Saji S, Zelada-Hedman M. Estrogen receptor beta in breast cancer. Endocrine Relat Canc. 2002;9(1):1.
Lindberg K, Ström A, Lock JG, Gustafsson JÖ, Haldosén LA, Helguero LA. Expression of estrogen receptor β increases integrin α1 and integrin β1 levels and enhances adhesion of breast cancer cells. J Cell Physiol. 2010;222(1):156–67.
Hartman J, Ström A, Gustafsson JÖ. Estrogen receptor beta in breast cancer–diagnostic and therapeutic implications. Steroids. 2009;74(8):635–41.
Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11.
Eden JA. Human breast cancer stem cells and sex hormones-a narrative review. Menopause. 2010;17(4):801.
Buzdar A, Jonat W, Howell A, Jones SE, Blomqvist C, Vogel CL, et al. Anastrozole, a potent and selective aromatase inhibitor, versus megestrol acetate in postmenopausal women with advanced breast cancer: results of overview analysis of two phase III trials. Arimidex Study Group. J Clin Oncol. 1996;14(7):2000.
Dombernowsky P, Smith I, Falkson G, Leonard R, Panasci L, Bellmunt J, et al. Letrozole, a new oral aromatase inhibitor for advanced breast cancer: double-blind randomized trial showing a dose effect and improved efficacy and tolerability compared with megestrol acetate. J Clin Oncol. 1998;16(2):453.
Kaufmann M, Bajetta E, Dirix LY, Fein LE, Jones SE, Zilembo N, et al. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. J Clin Oncol. 2000;18(7):1399.
Gershanovich M, Chaudri H, Campos D, Lurie H, Bonaventura A, Jeffrey M, et al. Letrozole, a new oral aromatase inhibitor: randomised trial comparing 2.5 mg daily, 0.5 mg daily and aminoglutethimide in postmenopausal women with advanced breast cancer. Ann Oncol. 1998;9(6):639.
Thurlimann B, Paridaens R, Serin D, Bonneterre J, Roche H, Murray R, et al. Third-line hormonal treatment with exemestane in postmenopausal patients with advanced breast cancer progressing on aminoglutethimide: a phase II multicentre multinational study. Eur J Cancer. 1997;33(11):1767–73.
Lonning PE, Bajetta E, Murray R, Tubiana-Hulin M, Eisenberg PD, Mickiewicz E, et al. Activity of exemestane in metastatic breast cancer after failure of nonsteroidal aromatase inhibitors: a phase II trial. J Clin Oncol. 2000;18(11):2234.
Thurlimann B, Robertson J, Nabholtz J, Buzdar A, Bonneterre J. Efficacy of tamoxifen following anastrozole (Arimidex) compared with anastrozole following tamoxifen as first-line treatment for advanced breast cancer in postmenopausal women. Eur J Cancer. 2003;39(16):2310–7.
Johnston S, Cheung K. Fulvestrant-a novel endocrine therapy for breast cancer. Curr Med Chem. 2010;17(10):902–14.
Valachis A, Mauri D, Polyzos NP, Mavroudis D, Georgoulias V, Casazza G. Fulvestrant in the treatment of advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Oncol/Hemat. 2010;73(3):220–7.
Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, et al. Results of the CONFIRM Phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594.
Peethambaram PP, Ingle JN, Suman VJ, Hartmann LC, Loprinzi CL. Randomized trial of diethylstilbestrol vs. tamoxifen in postmenopausal women with metastatic breast cancer. An updated analysis. Breast Canc Res Treat. 1999;54(2):117–22.
Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304(1):16–21.
Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302(7):774–80.
Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008;26(7):1059.
Rasmussen BB, Regan MM, Lykkesfeldt AE, Dell’Orto P, Del Curto B, Henriksen KL, et al. Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1-98 randomised trial. Lancet Oncol. 2008;9(1):23–8.
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2ñpositive, hormone receptorñpositive metastatic breast cancer: Results from the randomized phase III TAnDEM Study. J Clin Oncol. 2009;27(33):5529.
Johnston S, Pippen J, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptorñpositive metastatic breast cancer. J Clin Oncol. 2009;27(33):5538.
Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, et al. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16(5):1486.
Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9(6):667–76.
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500.
Kalinsky K, Jacks LM, Heguy A, Patil S, Drobnjak M, Bhanot UK, et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15(16):5049.
Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14(7):672–6.
Yu K, Toral-Barza L, Discafani C, Zhang W, Skotnicki J, Frost P, et al. mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocrine Relat Canc. 2001;8(3):249.
Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(Supplement 1):12.
Perez-Tenorio G, Karlsson E, Waltersson MA, Olsson B, Holmlund B et al. Clinical potential of the mTOR targets S6K1 and S6K2 in breast cancer. Breast Canc Res Treat. 2010:1–11.
Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278.
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptorñpositive breast cancer. J Clin Oncol. 2009;27(16):2630.
• Bourgier C, Ray-Coquard I, J. P, editors. Exploratory subgroup analysis of the TAMRAD phase 2 GINECO trial comparing tamoxifen (TAM) plus everolimus (RAD) with TAM alone in patients With Hormone-receptor–positive, HER2-negative Metastatic Breast Cancer (mBC) with prior exposure to aromatase inhibitors (AIs): implication for research strategies. Program and abstracts of the 2011 European Multidisciplinary Cancer Congress; 2011 September 23–27; Stockholm, Sweden. This abstract reports the results of the BOLERO-2 phase III trial.
•• Baselga J, Campone M, Piccart-Gebhart J, editors. Everolimus in combination with exemestane for postmenopausal women with advanced breast cancer who are refractory to letrozole or anastrozole: results of the BOLERO-2 phase III trial. Program and abstracts of the 2011 European Multidisciplinary Cancer Congress; 2011 September 23–27; Stockholm, Sweden. This is about the management of adverse events associated with the use of everolimus in advanced renal cell carcinoma, but it is useful for the breast cancer oncologist who wants to know more about the side effect profile of everolimus.
Porta C, Osanto S, Ravaud A, Climent MA, Vaishampayan U, White DA et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur J Canc. 2011.
Fagan D, Yee D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(4):423–9. doi:10.1007/s10911-008-9098-0.
Pritchard KI, Shepherd LE, Chapman J-AW, Norris BD, Cantin J, Goss PE, et al. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR therapy in the adjuvant treatment of early-stage breast cancer in postmenopausal women: NCIC CTG MA.14. J Clin Oncol. 2011;29(29):3869–76. doi:10.1200/jco.2010.33.7006.
Dickler MN. Targeting the insulin-like growth factor pathway in estrogen receptor positive breast cancer: a bumpy start with an uncertain future. J Clin Oncol. 2011;29(29):3846–8. doi:10.1200/jco.2011.36.6393.
O’ Shaughnessy J, Miles D, Gray R, Dieras V, Perez E, Zon R, et al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC). J Clin Oncol. 2010;28(15 suppl):1005.
Traina TA, Rugo HS, Caravelli JF, Patil S, Yeh B, Melisko ME, et al. Feasibility trial of letrozole in combination with bevacizumab in patients with metastatic breast cancer. J Clin Oncol. 2010;28(4):628–33. doi:10.1200/jco.2009.21.8784.
Roberts CG, Millar EKA, O’Toole SA, McNeil CM, Lehrbach GM, Pinese M, et al. Identification of PUMA as an estrogen target gene that mediates the apoptotic response to tamoxifen in human breast cancer cells and predicts patient outcome and tamoxifen responsiveness in breast cancer. Oncogene. 2011;30(28):3186–97.
Raha P, Thomas S, Munster PN. Epigenetic modulation: a novel therapeutic target for overcoming hormonal therapy resistance. Epigenomics. 2011;3(4):451–70.
Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir JM, Corbo L. Cracking the estrogen receptor’s posttranslational code in breast tumors. Endocrine Rev. 2011.
Munster P, Thurn K, Thomas S, Raha P, Lacevic M, Miller A, et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer. 2011;104(12):1828–35.
Robey RW, Chakraborty AR, Basseville A, Luchenko V, Bahr J, Zhan Z et al. Histone deacetylase inhibitors: emerging mechanisms of resistance. Mol Pharm. 2011.
Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, Sahin A, Liu S, Barrera JA, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10(6):1093.
Fernandez-Cuesta L, Anaganti S, Hainaut, Olivier. p53 status influences response to tamoxifen but not to fulvestrant in breast cancer cell lines. Int J Canc. 2010.
Weigelt B, Warne P, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene. 2011.
Disclosure
M. Khasraw is a consultant for Hoffman LaRoche and has received a research grant (paid to his institution) from Merck Serone. S.Harvey: none. R. Bell is a consultant for Novartis and has received a research grant (paid to his institution) from AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khasraw, M., Harvey, S.L. & Bell, R. Hormonal Resistance in Breast Cancer: Evolving Treatment Strategies. Curr Breast Cancer Rep 4, 66–74 (2012). https://doi.org/10.1007/s12609-011-0065-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-011-0065-1