Abstract
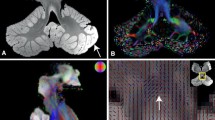
The dentate nucleus (DN) of the cerebellum is the major output nucleus of the cerebellum and is rich in iron. Quantitative susceptibility mapping (QSM) provides better iron-sensitive MRI contrast to delineate the boundary of the DN than either T2-weighted images or susceptibility-weighted images. Prior DN atlases used T2-weighted or susceptibility-weighted images to create DN atlases. Here, we employ QSM images to develop an improved dentate nucleus atlas for use in imaging studies. The DN was segmented in QSM images from 38 healthy volunteers. The resulting DN masks were transformed to a common space and averaged to generate the DN atlas. The center of mass of the left and right sides of the QSM-based DN atlas in the Montreal Neurological Institute space was −13.8, −55.8, and −36.4 mm, and 13.8, −55.7, and −36.4 mm, respectively. The maximal probability and mean probability of the DN atlas with the individually segmented DNs in this cohort were 100 and 39.3%, respectively, in contrast to the maximum probability of approximately 75% and the mean probability of 23.4 to 33.7% with earlier DN atlases. Using QSM, which provides superior iron-sensitive MRI contrast for delineating iron-rich structures, an improved atlas for the dentate nucleus has been generated. The atlas can be applied to investigate the role of the DN in both normal cortico-cerebellar physiology and the variety of disease states in which it is implicated.




Similar content being viewed by others
References
Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–2.
Koziol LF, Budding D, Andreasen N, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–77.
Koeppen AH, Ramirez RL, Yu D, et al. Friedreich’s ataxia causes redistribution of iron, copper, and zinc in the dentate nucleus. Cerebellum. 2012;11:845–60.
Pifl C, Schingnitz G, Hornykiewicz O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience. 1991;44:591–605.
Rolland AS, Herrero MT, Garcia-Martinez V, Ruberg M, Hirsch EC, Francois C. Metabolic activity of cerebellar and basal ganglia-thalamic neurons is reduced in parkinsonism. Brain. 2007;130:265–75.
Paris-Robidas S, Brochu E, Sintes M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135:105–16.
Marques JP, van der Zwaag W, Granziera C, Krueger G, Gruetter R. Cerebellar cortical layers: in vivo visualization with structural high-field-strength MR imaging. Radiology. 2010;254:942–8.
Deistung A, Stefanescu MR, Ernst TM, et al. Structural and functional magnetic resonance imaging of the cerebellum: considerations for assessing cerebellar ataxias. Cerebellum. 2016;15:21–5.
Li W, Wang N, Yu F, et al. A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. NeuroImage. 2015;108:111–22.
He N, Huang P, Ling H, et al. Dentate nucleus iron deposition is a potential biomarker for tremor-dominant Parkinson’s disease. NMR Biomed. 2016;30:e3554.
Solbach K, Kraff O, Minnerop M, et al. Cerebellar pathology in Friedreich’s ataxia: atrophied dentate nuclei with normal iron content. NeuroImage Clinical. 2014;6:93–9.
Dimitrova A, Zeljko D, Schwarze F, et al. Probabilistic 3D MRI atlas of the human cerebellar dentate/interposed nuclei. NeuroImage. 2006;30:12–25.
Diedrichsen J, Maderwald S, Kuper M, et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. NeuroImage. 2011;54:1786–94.
Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. NeuroImage. 2011;55:1645–56.
Langley J, Zhao Q. Unwrapping magnetic resonance phase maps with Chebyshev polynomials. Magn Reson Imaging. 2009;27:1293–301.
Haacke EM, Xu Y, Cheng Y-CN, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004;52:612–8.
Duvernoy HM. The human brain stem and cerebellum: surface, structure, vascularization, and three-dimensional sectional anatomy, with MRI. Springer Science & Business Media, 2012.
Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9:58–66.
Langley J, Huddleston DE, Merritt M, et al. Diffusion tensor imaging of the substantia nigra in Parkinson’s disease revisited. Hum Brain Mapp. 2016;37:2547–56.
Schafer A, Wharton S, Gowland P, Bowtell R. Using magnetic field simulation to study susceptibility-related phase contrast in gradient echo MRI. NeuroImage. 2009;48:126–37.
Dimitrova A, Weber J, Redies C, et al. MRI atlas of the human cerebellar nuclei. NeuroImage. 2002;17:240–55.
Höpker W. Das altern des nucleus dentatus. Z Altersforschung. 1951;5:256–77.
Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A, Yang S, Nestor PJ. In vivo MRI mapping of brain iron deposition across the adult lifespan. J Neurosci. 2016;36:364–74.
Maschke M, Weber J, Dimitrova A, et al. Age-related changes of the dentate nuclei in normal adults as revealed by 3D fast low angle shot (FLASH) echo sequence magnetic resonance imaging. J Neurol. 2004;251:740–6.
Louis ED, Rabinowitz D, Choe M, et al.. Mapping Purkinje cell placement along the Purkinje cell layer: an analysis of postmortem tissue from essential tremor patients vs. controls. Cerebellum 2015.
Ma H, Chen H, Fang J, et al. Resting-state functional connectivity of dentate nucleus is associated with tremor in Parkinson’s disease. J Neurol. 2015;262:2247–56.
Surova Y, Nilsson M, Latt J, et al. Disease-specific structural changes in thalamus and dentatorubrothalamic tract in progressive supranuclear palsy. Neuroradiology. 2015;57:1079–91.
Stefanescu MR, Dohnalek M, Maderwald S, et al. Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain. 2015;138:1182–97.
Hampson DR, Blatt GJ. Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci. 2015;9:420.
Acknowledgments
The authors thank Dr. Thomas Wichmann for the critical review of the manuscript. The authors thank Dr. Hongmei Fu and Dr. Hengfen Gong for the recruiting part of the volunteers. The authors wish to thank all the subjects who participated in this study. The study was supported in part through the State Key Clinical Department of Medical Imaging. XH, DH, and JL are supported in part by the Michael J. Fox Foundation (MJF 10854). CL is supported in part by the National Institutes of Health through grants (R01MH096979 and R01NS079653).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The current study was approved by local ethical committees. All subjects gave written informed consent in accordance with the Declaration of Helsinki in its currently applicable form.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
He, N., Langley, J., Huddleston, D.E. et al. Improved Neuroimaging Atlas of the Dentate Nucleus. Cerebellum 16, 951–956 (2017). https://doi.org/10.1007/s12311-017-0872-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0872-7