Abstract
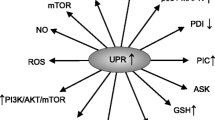
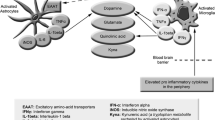
Nitric oxide plays an indispensable role in modulating cellular signaling and redox pathways. This role is mainly effected by the readily reversible nitrosylation of selective protein cysteine thiols. The reversibility and sophistication of this signaling system is enabled and regulated by a number of enzymes which form part of the thioredoxin, glutathione, and pyridoxine antioxidant systems. Increases in nitric oxide levels initially lead to a defensive increase in the number of nitrosylated proteins in an effort to preserve their function. However, in an environment of chronic oxidative and nitrosative stress (O&NS), nitrosylation of crucial cysteine groups within key enzymes of the thioredoxin, glutathione, and pyridoxine systems leads to their inactivation thereby disabling denitrosylation and transnitrosylation and subsequently a state described as “hypernitrosylation.” This state leads to the development of pathology in multiple domains such as the inhibition of enzymes of the electron transport chain, decreased mitochondrial function, and altered conformation of proteins and amino acids leading to loss of immune tolerance and development of autoimmunity. Hypernitrosylation also leads to altered function or inactivation of proteins involved in the regulation of apoptosis, autophagy, proteomic degradation, transcription factor activity, immune-inflammatory pathways, energy production, and neural function and survival. Hypernitrosylation, as a consequence of chronically elevated O&NS and activated immune-inflammatory pathways, can explain many characteristic abnormalities observed in neuroprogressive disease including major depression and chronic fatigue syndrome/myalgic encephalomyelitis. In those disorders, increased bacterial translocation may drive hypernitrosylation and autoimmune responses against nitrosylated proteins.


Similar content being viewed by others
References
Martinez-Ruiz A, Cadenas S, Lamas S (2011) Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic Biol Med 51(1):17–29. doi:10.1016/j.freeradbiomed.2011.04.010
Martinez-Ruiz A, Lamas S (2007) Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc Res 75(2):220–228. doi:10.1016/j.cardiores.2007.03.016
Ding H, Demple B (2000) Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proc Natl Acad Sci U S A 97(10):5146–5150
Hare JM, Stamler JS (2005) NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest 115(3):509–517. doi:10.1172/jci24459
Janssen-Heininger YMW, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG et al (2008) Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45(1):1–17. doi:10.1016/j.freeradbiomed.2008.03.011
Gould N, Doulias P-T, Tenopoulou M, Raju K, Ischiropoulos H (2013) Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J Biol Chem 288(37):26473–26479. doi:10.1074/jbc.R113.460261
Kovacs I, Lindermayr C (2013) Nitric oxide-based protein modification: formation and site-specificity of protein S-nitrosylation. Front Plant Sci 4:137. doi:10.3389/fpls.2013.00137
Lima B, Forrester MT, Hess DT, Stamler JS (2010) S-nitrosylation in cardiovascular signaling. Circ Res 106(4):633–646. doi:10.1161/circresaha.109.207381
Morris G, Maes M (2012) Increased nuclear factor-kappaB and loss of p53 are key mechanisms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med Hypotheses 79:607–613
Morris G, Maes M (2013) Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med 11:205
Nakamura T, Lipton SA (2013) Emerging role of protein-protein transnitrosylation in cell signaling pathways. Antioxid Redox Signal 18(3):239–249. doi:10.1089/ars.2012.4703
Gaston BM, Carver J, Doctor A, Palmer LA (2003) S-nitrosylation signaling in cell biology. Mol Interv 3(5):253–263. doi:10.1124/mi.3.5.253
Foster MW, Hess DT, Stamler JS (2009) Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15(9):391–404. doi:10.1016/j.molmed.2009.06.007
Benhar M, Forrester MT, Hess DT, Stamler JS (2008) Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320(5879):1050–1054. doi:10.1126/science.1158265
Lei SZ, Pan ZH, Aggarwal SK, Chen HS, Hartman J, Sucher NJ, Lipton SA (1992) Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron 8(6):1087–1099
Stamler J, Meissner G (2001) Physiology of nitric oxide in skeletal muscle. Physiol Rev 81:209–237
Marozkina NV, Gaston B (2012) S-nitrosylation signaling regulates cellular protein interactions. Biochim Biophys Acta 1820(6):722–729. doi:10.1016/j.bbagen.2011.06.017
Halloran M, Parakh S, Atkin JD (2013) The role of S-nitrosylation and S-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration. Int J Cell Biol. doi:10.1155/2013/797914
Anand P, Stamler JS (2012) Enzymatic mechanisms regulating protein S-nitrosylation: implications in health and disease. J Mol Med (Berl) 90(3):233–244. doi:10.1007/s00109-012-0878-z
Sun J, Murphy E (2010) Protein S-nitrosylation and cardioprotection. Circ Res 106(2):285–296. doi:10.1161/circresaha.109.209452
Wang G, Moniri NH, Ozawa K, Stamler JS, Daaka Y (2006) Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci U S A 103(5):1295–1300. doi:10.1073/pnas.0508354103
Seth D, Stamler JS (2011) The SNO-proteome: causation and classifications. Curr Opin Chem Biol 15(1):129–136. doi:10.1016/j.cbpa.2010.10.012
Okamoto S, Nakamura T, Cieplak P, Chan SF, Kalashnikova E, Liao L, Saleem S, Han X et al (2014) S-nitrosylation-mediated redox transcriptional switch modulates neurogenesis and neuronal cell death. Cell Rep 8(1):217–228. doi:10.1016/j.celrep.2014.06.005
Sun J, Steenbergen C, Murphy E (2006) S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal 8(9–10):1693–1705. doi:10.1089/ars.2006.8.1693
Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto S, Lipton SA (2013) Aberrant protein S-nitrosylation in neurodegenerative diseases. Neuron 78(4):596–614. doi:10.1016/j.neuron.2013.05.005
Nakamura T, Lipton SA (2007) S-nitrosylation and uncompetitive/fast off-rate (UFO) drug therapy in neurodegenerative disorders of protein misfolding. Cell Death Differ 14(7):1305–1314. doi:10.1038/sj.cdd.4402138
Piantadosi CA (2012) Regulation of mitochondrial processes by protein S-nitrosylation. Biochim Biophys Acta 1820(6):712–721. doi:10.1016/j.bbagen.2011.03.008
Shahani N, Sawa A (2011) Nitric oxide signaling and nitrosative stress in neurons: role for S-nitrosylation. Antioxid Redox Signal 14(8):1493–1504. doi:10.1089/ars.2010.3580
Hashemy SI, Holmgren A (2008) Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J Biol Chem 283(32):21890–21898. doi:10.1074/jbc.M801047200
Nakamura T, Lipton SA (2011) S-nitrosylation of critical protein thiols mediates protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Antioxid Redox Signal 14(8):1479–1492. doi:10.1089/ars.2010.3570
Nakamura T, Lipton SA (2011) Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases. Cell Death Differ 18(9):1478–1486
Shi ZQ, Sunico CR, McKercher SR, Cui J, Feng GS, Nakamura T, Lipton SA (2013) S-nitrosylated SHP-2 contributes to NMDA receptor-mediated excitotoxicity in acute ischemic stroke. Proc Natl Acad Sci U S A 110(8):3137–3142. doi:10.1073/pnas.1215501110
Qu J, Nakamura T, Cao G, Holland EA, McKercher SR, Lipton SA (2011) S-nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc Natl Acad Sci U S A 108(34):14330–14335. doi:10.1073/pnas.1105172108
Choi Y, Chen HV, Lipton SA (2001) Three pairs of cysteine residues mediate both redox and Zn2+ modulation of the NMDA receptor. J Neurosci 21(2):392–400
Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6(2):150–166. doi:10.1038/nrm1569
Boullerne A, Petry K, Geffard M (1996) Circulating antibodies directed against conjugated fatty acids in sera of patients with multiple sclerosis. J Neuroimmunol 65:75–81
Maes M, Mihaylova I, Leunis J (2006) Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol Lett 27:615–621
Maes M, Mihaylova I, Kubera M, Leunis JC, Geffard M (2011) IgM-mediated autoimmune responses directed against multiple neoepitopes in depression: new pathways that underpin the inflammatory and neuroprogressive pathophysiology. J Affect Disord 135(1–3):414–418. doi:10.1016/j.jad.2011.08.023
Maes M, Kubera M, Leunis JC, Berk M, Geffard M, Bosmans E (2013) In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr Scand 127(5):344–354. doi:10.1111/j.1600-0447.2012.01908.x
Hvaring C, Vujicic S, Aasly JO, Feinstein DL, White LR, Boullerne AI (2013) IgM to S-nitrosylated protein is found intrathecally in relapsing-remitting multiple sclerosis. J Neuroimmunol 256(1–2):77–83. doi:10.1016/j.jneuroim.2012.12.011
Morris G, Berk M, Walder K, Maes M (2015) Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med 13(1):28
Berk M, Williams L, Jacka F, O’Neil A, Pasco J, Moylan S (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 11:200
Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P (2012) Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10:66
Jourd’heuil FL, Lowery AM, Melton EM, Mnaimneh S, Bryan NS, Fernandez BO, Park JH, Ha CE et al (2010) Redox-sensitivity and site-specificity of S- and N-denitrosation in proteins. PLoS One 5(12), e14400. doi:10.1371/journal.pone.0014400
Viles K, Mathai C, Jourd’heuil FL, Jourd’heuil D (2013) Xanthine oxidase-mediated denitrosation of N-nitroso-tryptophan by superoxide and uric acid. Nitric Oxide: Biol Chem / Off J Nitric Oxide Soc 28:57–64. doi:10.1016/j.niox.2012.10.004
Suntsova TP, Beda NV, Nedospasov AA (2002) Structural features of proteins responsible for resistance of tryptophan residues to nitrosylation. IUBMB Life 54(5):281–292. doi:10.1080/15216540215677
Foster MW, Stamler JS (2004) New insights into protein S-nitrosylation. Mitochondria as a model system. J Biol Chem 279(24):25891–25897. doi:10.1074/jbc.M313853200
Jourd’heuil D, Jourd’heuil FL, Feelisch M (2003) Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem 278(18):15720–15726. doi:10.1074/jbc.M300203200
Evangelista AM, Kohr MJ, Murphy E (2013) S-nitrosylation: specificity, occupancy, and interaction with other post-translational modifications. Antioxid Redox Signal 19(11):1209–1219. doi:10.1089/ars.2012.5056
Garcia-Santos Mdel P, Gonzalez-Mancebo S, Hernandez-Benito J, Calle E, Casado J (2002) Reactivity of amino acids in nitrosation reactions and its relation to the alkylating potential of their products. J Am Chem Soc 124(10):2177–2182
Espey MG, Thomas DD, Miranda KM, Wink DA (2002) Focusing of nitric oxide mediated nitrosation and oxidative nitrosylation as a consequence of reaction with superoxide. Proc Natl Acad Sci U S A 99(17):11127–11132. doi:10.1073/pnas.152157599
Fernandez E, Garcia-Moreno JM, Martin de Pablos A, Chacon J (2013) May the evaluation of nitrosative stress through selective increase of 3-nitrotyrosine proteins other than nitroalbumin and dominant tyrosine-125/136 nitrosylation of serum alpha-synuclein serve for diagnosis of sporadic Parkinson’s disease? Antioxid Redox Signal 19(9):912–918. doi:10.1089/ars.2013.5250
Lee SJ, Lee JR, Kim YH, Park YS, Park SI, Park HS, Kim KP (2007) Investigation of tyrosine nitration and nitrosylation of angiotensin II and bovine serum albumin with electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 21(17):2797–2804. doi:10.1002/rcm.3145
Beda NV, Nedospasov AA (2001) Environment of tryptophan residues in proteins—a factor for stability to oxidative nitrosylation. I. Analysis of primary structure. Mol Genet Mikrobiol i Virusologiia 4:31–41
Kim WK, Choi YB, Rayudu PV, Das P, Asaad W, Arnelle DR, Stamler JS, Lipton SA (1999) Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO. Neuron 24(2):461–469
Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ et al (1993) A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364(6438):626–632. doi:10.1038/364626a0
Radi R (2013) Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res 46(2):550–559. doi:10.1021/ar300234c
Holzmeister C, Gaupels F, Geerlof A, Sarioglu H, Sattler M, Durner J, Lindermayr C (2015) Differential inhibition of Arabidopsis superoxide dismutases by peroxynitrite-mediated tyrosine nitration. J Exp Bot 66(3):989–999. doi:10.1093/jxb/eru458
Radi R (2004) Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A 101(12):4003–4008. doi:10.1073/pnas.0307446101
Castro L, Demicheli V, Tortora V, Radi R (2011) Mitochondrial protein tyrosine nitration. Free Radic Res 45(1):37–52. doi:10.3109/10715762.2010.516254
Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P et al (2011) Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med 208(10):1949–1962. doi:10.1084/jem.20101956
Yakovlev VA, Mikkelsen RB (2010) Protein tyrosine nitration in cellular signal transduction pathways. J Recept Signal Transduct Res 30(6):420–429. doi:10.3109/10799893.2010.513991
Torta F, Usuelli V, Malgaroli A, Bachi A (2008) Proteomic analysis of protein S-nitrosylation. Proteomics 8(21):4484–4494. doi:10.1002/pmic.200800089
Paige JS, Xu G, Stancevic B, Jaffrey SR (2008) Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem Biol 15(12):1307–1316. doi:10.1016/j.chembiol.2008.10.013
Hao G, Derakhshan B, Shi L, Campagne F, Gross SS (2006) SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci U S A 103(4):1012–1017. doi:10.1073/pnas.0508412103
Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T et al (2004) Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 116(4):617–628
Benhar M, Forrester MT, Stamler JS (2009) Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 10(10):721–732. doi:10.1038/nrm2764
Forrester MT, Seth D, Hausladen A, Eyler CE, Foster MW, Matsumoto A, Benhar M, Marshall HE et al (2009) Thioredoxin-interacting protein (Txnip) is a feedback regulator of S-nitrosylation. J Biol Chem 284(52):36160–36166. doi:10.1074/jbc.M109.057729
Jensen DE, Belka GK, Du Bois GC (1998) S-nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J 331(Pt 2):659–668
Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS (2001) A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410(6827):490–494. doi:10.1038/35068596
Holmgren A, Lu J (2010) Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun 396(1):120–124. doi:10.1016/j.bbrc.2010.03.083
Forrester MT, Foster MW, Stamler JS (2007) Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J Biol Chem 282(19):13977–13983. doi:10.1074/jbc.M609684200
Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA et al (2012) Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A 109(11):4314–4319. doi:10.1073/pnas.1113319109
Arner ES, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem / FEBS 267(20):6102–6109
Holmgren A (2000) Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2(4):811–820
Sengupta R, Ryter SW, Zuckerbraun BS, Tzeng E, Billiar TR, Stoyanovsky DA (2007) Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry 46(28):8472–8483. doi:10.1021/bi700449x
Stoyanovsky DA, Tyurina YY, Tyurin VA, Anand D, Mandavia DN, Gius D, Ivanova J, Pitt B et al (2005) Thioredoxin and lipoic acid catalyze the denitrosation of low molecular weight and protein S-nitrosothiols. J Am Chem Soc 127(45):15815–15823. doi:10.1021/ja0529135
Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y et al (2006) S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441(7092):513–517. doi:10.1038/nature04782
Wei W, Li B, Hanes MA, Kakar S, Chen X, Liu L (2010) S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci Transl Med 2(19):19ra13. doi:10.1126/scitranslmed.3000328
Haldar SM, Stamler JS (2013) S-nitrosylation: integrator of cardiovascular performance and oxygen delivery. J Clin Invest 123(1):101–110. doi:10.1172/jci62854
Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, Leonard B (2012) Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10:66. doi:10.1186/1741-7015-10-66
Fang J, Holmgren A (2006) Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J Am Chem Soc 128(6):1879–1885. doi:10.1021/ja057358l
Li H, Wan A, Xu G, Ye D (2013) Small changes huge impact: the role of thioredoxin 1 in the regulation of apoptosis by S-nitrosylation. Acta Biochim Biophys Sin 45(3):153–161. doi:10.1093/abbs/gms103
Wu C, Parrott AM, Fu C, Liu T, Marino SM, Gladyshev VN, Jain MR, Baykal AT et al (2011) Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxid Redox Signal 15(9):2565–2604. doi:10.1089/ars.2010.3831
Wu C, Liu T, Chen W, Oka S, Fu C, Jain MR, Parrott AM, Baykal AT et al (2010) Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol Cell Proteomics 9(10):2262–2275. doi:10.1074/mcp.M110.000034
Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M (2014) The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol 50:1059–1084
Rassaf T, Luedike P (2010) Between nitros(yl)ation and nitration: regulation of thioredoxin-1 in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol 49(3):343–346. doi:10.1016/j.yjmcc.2010.06.001
Yin T, Hou R, Liu S, Lau WB, Wang H, Tao L (2010) Nitrative inactivation of thioredoxin-1 increases vulnerability of diabetic hearts to ischemia/reperfusion injury. J Mol Cell Cardiol 49(3):354–361. doi:10.1016/j.yjmcc.2010.05.002
Wang YT, Piyankarage SC, Williams DL, Thatcher GR (2014) Proteomic profiling of nitrosative stress: protein S-oxidation accompanies S-nitrosylation. ACS Chem Biol 9(3):821–830. doi:10.1021/cb400547u
Sips PY, Irie T, Zou L, Shinozaki S, Sakai M, Shimizu N, Nguyen R, Stamler JS et al (2013) Reduction of cardiomyocyte S-nitrosylation by S-nitrosoglutathione reductase protects against sepsis-induced myocardial depression. Am J Physiol Heart Circ Physiol 304(8):H1134–1146. doi:10.1152/ajpheart.00887.2012
Das DK, Maulik N (2003) Preconditioning potentiates redox signaling and converts death signal into survival signal. Arch Biochem Biophys 420(2):305–311
Saini HK, Machackova J, Dhalla NS (2004) Role of reactive oxygen species in ischemic preconditioning of subcellular organelles in the heart. Antioxid Redox Signal 6(2):393–404. doi:10.1089/152308604322899468
Franco R, Cidlowski JA (2012) Glutathione efflux and cell death. Antioxid Redox Signal 17(12):1694–1713. doi:10.1089/ars.2012.4553
Hansen JM, Zhang H, Jones DP (2006) Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med 40(1):138–145. doi:10.1016/j.freeradbiomed.2005.09.023
Pias EK, Ekshyyan OY, Rhoads CA, Fuseler J, Harrison L, Aw TY (2003) Differential effects of superoxide dismutase isoform expression on hydroperoxide-induced apoptosis in PC-12 cells. J Biol Chem 278(15):13294–13301. doi:10.1074/jbc.M208670200
Cruz-Tapias P, Agmon-Levin N, Israeli E, Anaya JM, Shoenfeld Y (2013) Autoimmune (auto-inflammatory) syndrome induced by adjuvants (ASIA)—animal models as a proof of concept. Curr Med Chem 20(32):4030–4036
Garcia-Nogales P, Almeida A, Bolanos JP (2003) Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J Biol Chem 278(2):864–874. doi:10.1074/jbc.M206835200
Circu ML, Stringer S, Rhoads CA, Moyer MP, Aw TY (2009) The role of GSH efflux in staurosporine-induced apoptosis in colonic epithelial cells. Biochem Pharmacol 77(1):76–85. doi:10.1016/j.bcp.2008.09.011
Hammond CL, Madejczyk MS, Ballatori N (2004) Activation of plasma membrane reduced glutathione transport in death receptor apoptosis of HepG2 cells. Toxicol Appl Pharmacol 195(1):12–22. doi:10.1016/j.taap.2003.10.008
Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R (2006) The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem 97(2):373–384. doi:10.1111/j.1471-4159.2006.03737.x
Hirrlinger J, Dringen R (2005) Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol 400:395–409. doi:10.1016/s0076-6879(05)00023-6
Ballatori N, Krance SM, Marchan R, Hammond CL (2009) Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Asp Med 30(1–2):13–28. doi:10.1016/j.mam.2008.08.004
Franco R, Cidlowski JA (2006) SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem 281(40):29542–29557. doi:10.1074/jbc.M602500200
Nakamura T, Prikhodko OA, Pirie E, Nagar S, Akhtar MW, Oh CK, McKercher SR, Ambasudhan R et al (2015) Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiol Dis. doi:10.1016/j.nbd.2015.03.017
Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC et al (2002) S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297(5584):1186–1190. doi:10.1126/science.1073634
Rhee SG, Chae HZ, Kim K (2005) Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38(12):1543–1552. doi:10.1016/j.freeradbiomed.2005.02.026
Krapfenbauer K, Engidawork E, Cairns N, Fountoulakis M, Lubec G (2003) Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res 967(1–2):152–160
Sarafian TA (1999) Methylmercury-induced generation of free radicals: biological implications. Met Ions Biol Syst 36:415–444
Romero-Puertas MC, Laxa M, Matte A, Zaninotto F, Finkemeier I, Jones AM, Perazzolli M, Vandelle E et al (2007) S-nitrosylation of peroxiredoxin II E promotes peroxynitrite-mediated tyrosine nitration. Plant Cell 19(12):4120–4130. doi:10.1105/tpc.107.055061
Gu Z, Nakamura T, Lipton SA (2010) Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol Neurobiol 41(2–3):55–72. doi:10.1007/s12035-010-8113-9
Walker AK, Farg MA, Bye CR, McLean CA, Horne MK, Atkin JD (2010) Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain 133(Pt 1):105–116. doi:10.1093/brain/awp267
Conn KJ, Gao W, McKee A, Lan MS, Ullman MD, Eisenhauer PB, Fine RE, Wells JM (2004) Identification of the protein disulfide isomerase family member PDIp in experimental Parkinson’s disease and Lewy body pathology. Brain Res 1022(1–2):164–172. doi:10.1016/j.brainres.2004.07.026
Landau G, Kodali VK, Malhotra JD, Kaufman RJ (2013) Detection of oxidative damage in response to protein misfolding in the endoplasmic reticulum. Methods Enzymol 526:231–250. doi:10.1016/b978-0-12-405883-5.00014-4
Ali Khan H, Mutus B (2014) Protein disulfide isomerase a multifunctional protein with multiple physiological roles. Front Chem 2:70. doi:10.3389/fchem.2014.00070
Chung K, Thomas B, Li X, Pletnikova O, Troncoso J, Marsh L (2004) S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science 304:1328–1331
Yao D, Gu Z, Nakamura T, Shi Z, Ma Y, Gaston B (2004) Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A 101:10810–10814
Meng F, Yao D, Shi Y, Kabakoff J, Wu W, Reicher J, Ma Y, Moosmann B et al (2011) Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol Neurodegener 6:34. doi:10.1186/1750-1326-6-34
Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE (2007) NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem 282(42):30667–30672. doi:10.1074/jbc.M705929200
dela Torre A, Schroeder RA, Bartlett ST, Kuo PC (1998) Differential effects of nitric oxide-mediated S-nitrosylation on p50 and c-jun DNA binding. Surgery 124(2):137–141, discussion 141-132
Delatorre A, Schroeder RA, Kuo PC (1997) Alteration of NF-kB p50 DNA binding kinetics by S-nitrosylation. Biochem Biophys Res Commun 238(3):703–706. doi:10.1006/bbrc.1997.7279
Matthews JR, Botting CH, Panico M, Morris HR, Hay RT (1996) Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res 24(12):2236–2242
Marshall HE, Stamler JS (2001) Inhibition of NF-kappa B by S-nitrosylation. Biochemistry 40(6):1688–1693
Park SK, Lin HL, Murphy S (1997) Nitric oxide regulates nitric oxide synthase-2 gene expression by inhibiting NF-kappaB binding to DNA. Biochem J 322(Pt 2):609–613
Marshall HE, Hess DT, Stamler JS (2004) S-nitrosylation: physiological regulation of NF-kappaB. Proc Natl Acad Sci U S A 101(24):8841–8842. doi:10.1073/pnas.0403034101
Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM (2004) Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A 101(24):8945–8950. doi:10.1073/pnas.0400588101
Morris G, Maes M (2012) A neuro-immune model of myalgic encephalomyelitis/chronic fatigue syndrome. Metab Brain Dis 28(4):523–540
Anderson G, Berk M, Dean O, Moylan S, Maes M (2014) Role of immune-inflammatory and oxidative and nitrosative stress pathways in the etiology of depression: therapeutic implications. CNS Drugs 28(1):1–10. doi:10.1007/s40263-013-0119-1
Adib-Conquy M, Cavaillon JM (2009) Compensatory anti-inflammatory response syndrome. Thromb Haemost 101(1):36–47
Ward NS, Casserly B, Ayala A (2008) The compensatory anti-inflammatory response syndrome (CARS) in Critically ill patients. Clin Chest Med 29 (4):617-viii. doi:10.1016/j.ccm.2008.06.010
Huston JM (2012) The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis. Surg Infect 13(4):187–193. doi:10.1089/sur.2012.126
Martelli D, McKinley MJ, McAllen RM (2014) The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci: Basic Clin 182:65–69. doi:10.1016/j.autneu.2013.12.007
Hutchins AP, Diez D, Miranda-Saavedra D (2013) The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics 12(6):489–498. doi:10.1093/bfgp/elt028
Bazzoni F, Tamassia N, Rossato M, Cassatella MA (2010) Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: lessons from neutrophils. Eur J Immunol 40(9):2360–2368. doi:10.1002/eji.200940294
Campbell DJ, Koch MA (2011) Phenotypic and functional specialization of FOXP3(+) regulatory T cells. Nat Rev Immunol 11(2):119–130. doi:10.1038/nri2916
Hayden MS, West AP, Ghosh S (2006) NF-[kappa]B and the immune response. Oncogene 25(51):6758–6780
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5(10):749–759. doi:10.1038/nri1703
Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl):S81–96
Xiong H, Zhu C, Li F, Hegazi R, He K, Babyatsky M, Bauer AJ, Plevy SE (2004) Inhibition of interleukin-12 p40 transcription and NF-kappaB activation by nitric oxide in murine macrophages and dendritic cells. J Biol Chem 279(11):10776–10783. doi:10.1074/jbc.M313416200
Schroeder RA, Cai C, Kuo PC (1999) Endotoxin-mediated nitric oxide synthesis inhibits IL-1β gene transcription in ANA-1 murine macrophages. Am J Physiol Cell Physiol 277(3):C523–C530
Yu Z, Kuncewicz T, Dubinsky WP, Kone BC (2006) Nitric oxide-dependent negative feedback of PARP-1 trans-activation of the inducible nitric-oxide synthase gene. J Biol Chem 281(14):9101–9109. doi:10.1074/jbc.M511049200
Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, Ayasolla K, Elango C, Singh AK et al (2005) S-nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab 25(2):177–192. doi:10.1038/sj.jcbfm.9600012
Gonzalez-Leon MC, Soares-Schanoski A, del Fresno C, Cimadevila A, Gomez-Pina V, Mendoza-Barbera E, Garcia F, Marin E et al (2006) Nitric oxide induces SOCS-1 expression in human monocytes in a TNF-alpha-dependent manner. J Endotoxin Res 12(5):296–306. doi:10.1179/096805106x118843
del Fresno C, Gomez-Garcia L, Caveda L, Escoll P, Arnalich F, Zamora R, Lopez-Collazo E (2004) Nitric oxide activates the expression of IRAK-M via the release of TNF-alpha in human monocytes. Nitric Oxide: Biol Chem / Off J Nitric Oxide Soc 10(4):213–220. doi:10.1016/j.niox.2004.04.007
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58(11):621–631. doi:10.1080/15216540600957438
Dobreva ZG, Miteva LD, Stanilova SA (2009) The inhibition of JNK and p38 MAPKs downregulates IL-10 and differentially affects c-Jun gene expression in human monocytes. Immunopharmacol Immunotoxicol 31(2):195–201. doi:10.1080/08923970802626276
Dong C, Davis RJ, Flavell RA (2002) MAP kinases in the immune response. Annu Rev Immunol 20:55–72. doi:10.1146/annurev.immunol.20.091301.131133
Manetsch M, Seidel P, Heintz U, Che W, Hughes JM, Ge Q, Sukkar MB, Ammit AJ (2012) TLR2 ligand engagement upregulates airway smooth muscle TNFalpha-induced cytokine production. Am J Physiol Lung Cell Mol Physiol 302(9):L838–845. doi:10.1152/ajplung.00317.2011
Tegeder I, Scheving R, Wittig I, Geisslinger G (2011) SNO-ing at the nociceptive synapse? Pharmacol Rev 63(2):366–389. doi:10.1124/pr.110.004200
Park HS, Huh SH, Kim MS, Lee SH, Choi EJ (2000) Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc Natl Acad Sci U S A 97(26):14382–14387. doi:10.1073/pnas.97.26.14382
Lander HM, Ogiste JS, Pearce SF, Levi R, Novogrodsky A (1995) Nitric oxide-stimulated guanine nucleotide exchange on p21ras. J Biol Chem 270(13):7017–7020
Nikitovic D, Holmgren A, Spyrou G (1998) Inhibition of AP-1 DNA binding by nitric oxide involving conserved cysteine residues in Jun and Fos. Biochem Biophys Res Commun 242(1):109–112. doi:10.1006/bbrc.1997.7930
Qi SH, Hao LY, Yue J, Zong YY, Zhang GY (2013) Exogenous nitric oxide negatively regulates the S-nitrosylation p38 mitogen-activated protein kinase activation during cerebral ischaemia and reperfusion. Neuropathol Appl Neurobiol 39(3):284–297. doi:10.1111/j.1365-2990.2012.01284.x
Hess DT, Stamler JS (2012) Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem 287(7):4411–4418. doi:10.1074/jbc.R111.285742
Medvedev AE, Piao W (2009) Analysis of the functional role of Toll-like receptor-4 tyrosine phosphorylation. Methods Mol Biol 517:145–167. doi:10.1007/978-1-59745-541-1_10
Johnson P, Cross JL (2009) Tyrosine phosphorylation in immune cells: direct and indirect effects on toll-like receptor-induced proinflammatory cytokine production. Crit Rev Immunol 29(4):347–367
Caselli A, Chiarugi P, Camici G, Manao G, Ramponi G (1995) In vivo inactivation of phosphotyrosine protein phosphatases by nitric oxide. FEBS Lett 374(2):249–252
Callsen D, Sandau KB, Brune B (1999) Nitric oxide and superoxide inhibit platelet-derived growth factor receptor phosphotyrosine phosphatases. Free Radic Biol Med 26(11–12):1544–1553
Li S, Whorton AR (2003) Regulation of protein tyrosine phosphatase 1B in intact cells by S-nitrosothiols. Arch Biochem Biophys 410(2):269–279
Mikkelsen RB, Wardman P (2003) Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 22(37):5734–5754. doi:10.1038/sj.onc.1206663
Ryan KA, Smith MF Jr, Sanders MK, Ernst PB (2004) Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun 72(4):2123–2130
Yuan X, Zhou Y, Wang W, Li J, Xie G, Zhao Y, Xu D, Shen L (2013) Activation of TLR4 signaling promotes gastric cancer progression by inducing mitochondrial ROS production. Cell Death Dis 4, e794. doi:10.1038/cddis.2013.334
Chen YJ, Lu CT, Su MG, Huang KY, Ching WC, Yang HH, Liao YC, Chen YJ et al (2015) dbSNO 2.0: a resource for exploring structural environment, functional and disease association and regulatory network of protein S-nitrosylation. Nucleic Acids Res 43(Database issue):D503–511. doi:10.1093/nar/gku1176
Lucas K, Maes M (2013) Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol 48:190–204
Lucas K, Morris G, Anderson G, Maes M (2015) The Toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS Neurol Disord Drug Targets 14(7):838–854
Uchida K (2013) Redox-derived damage-associated molecular patterns: ligand function of lipid peroxidation adducts. Redox Biol 1(1):94–96. doi:10.1016/j.redox.2012.12.005
Moghaddam AE, Gartlan KH, Kong L, Sattentau QJ (2011) Reactive carbonyls are a major Th2-inducing damage-associated molecular pattern generated by oxidative stress. J Immunol 187(4):1626–1633. doi:10.4049/jimmunol.1003906
Morris G, Berk M (2015) The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med 13(1):68
Land WG (2015) The role of damage-associated molecular patterns in human diseases: part I—promoting inflammation and immunity. Sultan Qaboos Univ Med J 15(1):e9–e21
Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA et al (2008) Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57(10):2595–2602. doi:10.2337/db08-0038
Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW et al (2007) Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100(11):1589–1596. doi:10.1161/circresaha.106.142851
Morris G, Maes M (2014) Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr Neuropharmacol 12:168–185
Into T, Inomata M, Nakashima M, Shibata K, Hacker H, Matsushita K (2008) Regulation of MyD88-dependent signaling events by S nitrosylation retards toll-like receptor signal transduction and initiation of acute-phase immune responses. Mol Cell Biol 28(4):1338–1347. doi:10.1128/mcb.01412-07
Panaro MA, Gagliardi N, Saponaro C, Calvello R, Mitolo V, Cianciulli A (2010) Toll-like receptor 4 mediates LPS-induced release of nitric oxide and tumor necrosis factor-alpha by embryonal cardiomyocytes: biological significance and clinical implications in human pathology. Curr Pharm Des 16(7):766–774
Mizel SB, Honko AN, Moors MA, Smith PS, West AP (2003) Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J Immunol 170(12):6217–6223
Zheng Z-k, Wang J-j, Hu H, Jiang K, Nie J, Zhang J, Guo H, X-w Q et al (2013) Short-term inhalation of nitric oxide inhibits activations of toll-like receptor 2 and 4 in the lung after ischemia-reperfusion injury in mice. J Huazhong Univ Sci Technol [Med Sci] 33(2):219–223. doi:10.1007/s11596-013-1100-4
Um HC, Jang JH, Kim DH, Lee C, Surh YJ (2011) Nitric oxide activates Nrf2 through S-nitrosylation of Keap1 in PC12 cells. Nitric Oxide: Biol Chem / Off J Nitric Oxide Soc 25(2):161–168. doi:10.1016/j.niox.2011.06.001
Fourquet S, Guerois R, Biard D, Toledano MB (2010) Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem 285(11):8463–8471. doi:10.1074/jbc.M109.051714
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12(12):931–947. doi:10.1038/nrd4002
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284(20):13291–13295. doi:10.1074/jbc.R900010200
Jaramillo MC, Zhang DD (2013) The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev 27(20):2179–2191. doi:10.1101/gad.225680.113
Kim J, Cha YN, Surh YJ (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690(1–2):12–23. doi:10.1016/j.mrfmmm.2009.09.007
Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN (2008) Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76(11):1485–1489. doi:10.1016/j.bcp.2008.07.017
Hwang JW, Yao H, Caito S, Sundar IK, Rahman I (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61:95–110. doi:10.1016/j.freeradbiomed.2013.03.015
Moffet J, Namboodiri M (2003) Tryptophan and the immune response. Immunol Cell Biol 81:247–265
Salminen A, Kauppinen A, Suuronen T, Kaarniranta K (2008) SIRT1 longevity factor suppresses NF-kB-driven immune responses: regulation of aging via NF-kB acetylation? BioEssays 30(10):939–942. doi:10.1002/bies.20799
Shinozaki S, Chang K, Sakai M, Shimizu N, Yamada M, Tanaka T, Nakazawa H, Ichinose F et al (2014) Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci Signal 7(351):ra106. doi:10.1126/scisignal.2005375
Menendez D, Shatz M, Resnick MA (2013) Interactions between the tumor suppressor p53 and immune responses. Curr Opin Oncol 25(1):85–92. doi:10.1097/CCO.0b013e32835b6386
Raj N, Attardi Laura D (2013) Tumor suppression: p53 alters immune surveillance to restrain liver cancer. Curr Biol 23(12):R527–R530. doi:10.1016/j.cub.2013.04.076
Shatz M, Menendez D, Resnick MA (2012) The human TLR innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res 72(16):3948–3957. doi:10.1158/0008-5472.can-11-4134
Maes M, Kubera M, Mihaylova I, Geffard M, Galecki P, Leunis J (2013) Increased autoimmune responses against auto-epitopes modified by oxidative and nitrosative damage in depression: implications for the pathways to chronic depression and neuroprogression. J Affect Disord 149:23–29
Lutz HU (2007) Homeostatic roles of naturally occurring antibodies: an overview. J Autoimmun 29(4):287–294. doi:10.1016/j.jaut.2007.07.007
Jones DD, DeIulio GA, Winslow GM (2012) Antigen-driven induction of polyreactive IgM during intracellular bacterial infection. J Immunol 189(3):1440–1447. doi:10.4049/jimmunol.1200878
Chadebech P, Michel M, Janvier D, Yamada K, Copie-Bergman C, Bodivit G, Bensussan A, Fournie JJ et al (2010) IgA-mediated human autoimmune hemolytic anemia as a result of hemagglutination in the spleen, but independent of complement activation and FcalphaRI. Blood 116(20):4141–4147. doi:10.1182/blood-2010-03-276162
Tsiantoulas D, Gruber S, Binder CJ (2012) B-1 cell immunoglobulin directed against oxidation-specific epitopes. Front Immunol 3:415. doi:10.3389/fimmu.2012.00415
Weismann D, Binder CJ (2012) The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta 1818(10):2465–2475. doi:10.1016/j.bbamem.2012.01.018
Baumgarth N (2011) The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 11(1):34–46. doi:10.1038/nri2901
Binder CJ (2010) Natural IgM antibodies against oxidation-specific epitopes. J Clin Immunol 30(Suppl 1):S56–60. doi:10.1007/s10875-010-9396-3
Binder CJ, Silverman GJ (2005) Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol 26(4):385–404. doi:10.1007/s00281-004-0185-z
Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J (2000) Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A 97(3):1184–1189
Zhang M, Carroll MC (2007) Natural antibody mediated innate autoimmune response. Mol Immunol 44(1–3):103–110. doi:10.1016/j.molimm.2006.06.022
Haas MS, Alicot EM, Schuerpf F, Chiu I, Li J, Moore FD, Carroll MC (2010) Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res 87(4):618–627. doi:10.1093/cvr/cvq141
Boullerne AI, Rodriguez JJ, Touil T, Brochet B, Schmidt S, Abrous ND, Le Moal M, Pua JR et al (2002) Anti-S-nitrosocysteine antibodies are a predictive marker for demyelination in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neurosci 22(1):123–132
Semballa S, Geffard M, Daulouede S, Malvy D, Veyret B, Lemesre JL, Holzmuller P, Mnaimneh S et al (2004) Antibodies directed against nitrosylated neoepitopes in sera of patients with human African trypanosomiasis. Tropical Med Int Health 9(10):1104–1110. doi:10.1111/j.1365-3156.2004.01305.x
Bodet D, Glaize G, Dabadie MP, Geffard M (2004) Suivi immunobiologique de malades atteints de sclérose en plaques. Immuno-analyse Biol Spécialisée 19(3):138–147. doi:10.1016/j.immbio.2004.03.007
Marrie RA, Cohen J, Stuve O, Trojano M, Sørensen PS, Reingold S, Cutter G, Reider N (2015) A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Multiple Scler 21(3):263–281. doi:10.1177/1352458514564491
Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A et al (2015) Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One 10(9), e0137429. doi:10.1371/journal.pone.0137429
Maes M, Leunis JC, Geffard M, Berk M (2014) Evidence for the existence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) with and without abdominal discomfort (irritable bowel) syndrome. Neuro Endocrinol Lett 35(6):445–453
Maes M (2009) Inflammatory and oxidative and nitrosative stress pathways underpinning chronic fatigue, somatization and psychosomatic symptoms. Curr Opin Psychiatry 22(1):75–83
Maes M (2015) A new case definition of neuro-inflammatory and oxidative fatigue (NIOF), a neuroprogressive disorder, formerly known as chronic fatigue syndrome or myalgic encephalomyelitis: results of multivariate pattern recognition methods and external validation by neuro-immune biomarkers. Neuro Endocrinol Lett 36(4):320–329
Maes M, Noto C, Brietzke E (2015) Omics-based depression and inflammation research. Rev Bras Psiquiatr 37:1–2
Doulias PT, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H (2013) Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci Signal 6(256):rs1. doi:10.1126/scisignal.2003252
Mailloux RJ, Jin X, Willmore WG (2014) Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol 2:123–139. doi:10.1016/j.redox.2013.12.011
Drose S, Brandt U, Wittig I (2014) Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim Biophys Acta 1844(8):1344–1354. doi:10.1016/j.bbapap.2014.02.006
Chang AH, Sancheti H, Garcia J, Kaplowitz N, Cadenas E, Han D (2014) Respiratory substrates regulate S-nitrosylation of mitochondrial proteins through a thiol-dependent pathway. Chem Res Toxicol 27(5):794–804. doi:10.1021/tx400462r
Murray CI, Uhrigshardt H, O’Meally RN, Cole RN, Van Eyk JE (2012) Identification and quantification of S-nitrosylation by cysteine reactive tandem mass tag switch assay. Mol Cell Proteomics 11(2):M111.013441. doi:10.1074/mcp.M111.013441
Clementi E, Nisoli E (2005) Nitric oxide and mitochondrial biogenesis: a key to long-term regulation of cellular metabolism. Comp Biochem Physiol A Mol Integr Physiol 142(2):102–110. doi:10.1016/j.cbpb.2005.04.022
Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS (2009) In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol 46(6):960–968. doi:10.1016/j.yjmcc.2009.01.012
Nadtochiy SM, Burwell LS, Brookes PS (2007) Cardioprotection & mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol 42(4):812–825. doi:10.1016/j.yjmcc.2007.01.010
Jneid H, Chandra M, Alshaher M, Hornung CA, Tang XL, Leesar M, Bolli R (2005) Delayed preconditioning-mimetic actions of nitroglycerin in patients undergoing exercise tolerance tests. Circulation 111(20):2565–2571. doi:10.1161/circulationaha.104.515445
Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP et al (2009) A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A 106(26):10764–10769. doi:10.1073/pnas.0903250106
Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E (2007) Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 101(11):1155–1163. doi:10.1161/circresaha.107.155879
Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH et al (2007) Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204(9):2089–2102. doi:10.1084/jem.20070198
Galkin A, Moncada S (2007) S-nitrosation of mitochondrial complex I depends on its structural conformation. J Biol Chem 282:37448–37453
Clementi E, Brown G, Feelisch M, Moncada S (1998) Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A 95:7631–7636
Sarti P, Arese M, Forte E, Giuffre A, Mastronicola D (2012) Mitochondria and nitric oxide: chemistry and pathophysiology. Adv Exp Med Biol 942:75–92. doi:10.1007/978-94-007-2869-1_4
Sarti P, Arese M, Giuffre A (2003) The molecular mechanisms by which nitric oxide controls mitochondrial complex IV. Ital J Biochem 52(1):37–42
Zhang J, Jin B, Li L, Block E, Patel J (2005) Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am J Physiol Cell Physiol 288:C840–849
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328(2):309–316. doi:10.1006/abbi.1996.0178
Batthyany C, Souza J, Duran R, Cassina A, Cervenansky C, Radi R (2005) Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry 44:8038–8046
Poderoso JJ, Lisdero C, Schopfer F, Riobo N, Carreras MC, Cadenas E, Boveris A (1999) The regulation of mitochondrial oxygen uptake by redox reactions involving nitric oxide and ubiquinol. J Biol Chem 274(53):37709–37716
Poderoso J, Carreras M, Lisdero C, Riobo N, Schopfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
Nakagawa D, Ohshima Y, Takusagawa M, Ikota N, Takahashi Y, Shimizu S, Ozawa T (2001) Functional modification of cytochrome c by peroxynitrite in an electron transfer reaction. Chem Pharm Bull 49(12):1547–1554
Jang B, Han S (2006) Biochemical properties of cytochrome c nitrated by peroxynitrite. Biochimie 88:53–58
Larson SK, Dwyer D, Lo HH, Ghafourifar P (2006) Mitochondrial cytochrome c reacts with nitric oxide via S-nitrosation. Biochem Biophys Res Commun 342(3):991–995
Javadov S, Kuznetsov A (2013) Mitochondrial permeability transition and cell death: the role of cyclophilin D. Front Physiol 4:76. doi:10.3389/fphys.2013.00076
Han D, Canali R, Garcia J, Aguilera R, Gallaher T, Cadenas E (2005) Sites and mechanisms of aconitase inactivation by peroxynitrite: modulation by citrate and glutathione. Biochemistry 44:11986–11996
Gardner PR, Costantino G, Szabó C, Salzman AL (1997) Nitric oxide sensitivity of the aconitases. J Biol Chem 272(40):25071–25076. doi:10.1074/jbc.272.40.25071
Kaasik A, Minajeva A, De Sousa E, Ventura-Clapier R, Veksler V (1999) Nitric oxide inhibits cardiac energy production via inhibition of mitochondrial creatine kinase. FEBS Lett 444(1):75–77
Gross WL, Bak MI, Ingwall JS, Arstall MA, Smith TW, Balligand JL, Kelly RA (1996) Nitric oxide inhibits creatine kinase and regulates rat heart contractile reserve. Proc Natl Acad Sci U S A 93(11):5604–5609
Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C et al (2005) S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron 46(4):533–540. doi:10.1016/j.neuron.2005.03.028
Selvakumar B, Huganir RL, Snyder SH (2009) S-nitrosylation of stargazin regulates surface expression of AMPA-glutamate neurotransmitter receptors. Proc Natl Acad Sci U S A 106(38):16440–16445. doi:10.1073/pnas.0908949106
Lu J, Katano T, Okuda-Ashitaka E, Oishi Y, Urade Y, Ito S (2009) Involvement of S-nitrosylation of actin in inhibition of neurotransmitter release by nitric oxide. Mol Pain 5:58. doi:10.1186/1744-8069-5-58
Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S et al (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2(11):596–607. doi:10.1038/nchembio821
Jian K, Chen M, Cao X, Zhu XH, Fung ML, Gao TM (2007) Nitric oxide modulation of voltage-gated calcium current by S-nitrosylation and cGMP pathway in cultured rat hippocampal neurons. Biochem Biophys Res Commun 359(3):481–485. doi:10.1016/j.bbrc.2007.05.113
Asada K, Kurokawa J, Furukawa T (2009) Redox- and calmodulin-dependent S-nitrosylation of the KCNQ1 channel. J Biol Chem 284(9):6014–6020. doi:10.1074/jbc.M807158200
Palmer ZJ, Duncan RR, Johnson JR, Lian LY, Mello LV, Booth D, Barclay JW, Graham ME et al (2008) S-nitrosylation of syntaxin 1 at Cys(145) is a regulatory switch controlling Munc18-1 binding. Biochem J 413(3):479–491. doi:10.1042/bj20080069
Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11(10):682–696. doi:10.1038/nrn2911
Nakamura T, Lipton SA (2015) Nitrosative stress in the nervous system: guidelines for designing experimental strategies to study protein S-nitrosylation. Neurochem Res. doi:10.1007/s11064-015-1640-z
Gama CS, Kunz M, Magalhaes PV, Kapczinski F (2013) Staging and neuroprogression in bipolar disorder: a systematic review of the literature. Rev Bras Psiquiatr 35(1):70–74
Maes M, Kubera M, Mihaylova I, Geffard M, Galecki P, Leunis JC, Berk M (2013) Increased autoimmune responses against auto-epitopes modified by oxidative and nitrosative damage in depression: implications for the pathways to chronic depression and neuroprogression. J Affect Disord 149(1–3):23–29. doi:10.1016/j.jad.2012.06.039
Berk M, Kapczinski F, Andreazza A, Dean O, Giorlando F, Maes M (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35:804–817
Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL et al (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 11:200. doi:10.1186/1741-7015-11-200
Moylan S, Maes M, Wray NR, Berk M (2013) The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18(5):595–606. doi:10.1038/mp.2012.33
Shimizu NK, Hiroyuki, Kaneki M (2007) S-nitrosylation-mediated activation of glycogen synthase kinase-3 β and its reversal by S-nitrosoglutathione (GSNO) reductase (GSNOR). Diabetes 56(1):330–331
Feng Y, Xia Y, Yu G, Shu X, Ge H, Zeng K, Wang J, Wang X (2013) Cleavage of GSK-3beta by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3beta activity induced by H(2)O(2). J Neurochem 126(2):234–242. doi:10.1111/jnc.12285
Chiara F, Gambalunga A, Sciacovelli M, Nicolli A, Ronconi L, Fregona D, Bernardi P, Rasola A et al (2012) Chemotherapeutic induction of mitochondrial oxidative stress activates GSK-3alpha/beta and Bax, leading to permeability transition pore opening and tumor cell death. Cell Death Dis 3, e444. doi:10.1038/cddis.2012.184
Avrahami L, Eldar-Finkelman H (2013) GSK-3 and lysosomes meet in Alzheimer’s disease. Commun Integr Biol 6(5), e25179
Avrahami L, Farfara D, Shaham-Kol M, Vassar R, Frenkel D, Eldar-Finkelman H (2013) Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J Biol Chem 288(2):1295–1306. doi:10.1074/jbc.M112.409250
Beurel E (2011) Regulation by glycogen synthase kinase-3 of inflammation and T cells in CNS diseases. Front Mol Neurosci 4:18. doi:10.3389/fnmol.2011.00018
Beurel E, Michalek SM, Jope RS (2010) Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol 31(1):24. doi:10.1016/j.it.2009.09.007
Hofmann C, Dunger N, Scholmerich J, Falk W, Obermeier F (2010) Glycogen synthase kinase 3-beta: a master regulator of toll-like receptor-mediated chronic intestinal inflammation. Inflamm Bowel Dis 16(11):1850–1858. doi:10.1002/ibd.21294
Wang H, Brown J, Martin M (2011) Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine 53(2):130–140. doi:10.1016/j.cyto.2010.10.009
Huang WC, Lin YS, Wang CY, Tsai CC, Tseng HC, Chen CL, Lu PJ, Chen PS et al (2009) Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology 128(1 Suppl):e275–286. doi:10.1111/j.1365-2567.2008.02959.x
Okamoto S, Lipton SA (2015) S-nitrosylation in neurogenesis and neuronal development. Biochim Biophys Acta 1850(8):1588–1593. doi:10.1016/j.bbagen.2014.12.013
Libert S, Cohen D, Guarente L (2008) Neurogenesis directed by Sirt1. Nat Cell Biol 10(4):373–374
Vithlani M, Hines RM, Zhong P, Terunuma M, Hines DJ, Revilla-Sanchez R, Jurd R, Haydon P et al (2013) The ability of BDNF to modify neurogenesis and depressive-like behaviors is dependent upon phosphorylation of tyrosine residues 365/367 in the GABAA-receptor γ2 subunit. J Neurosci 33(39):15567–15577. doi:10.1523/jneurosci.1845-13.2013
Blazquez C, Chiarlone A, Bellocchio L, Resel E, Pruunsild P, Garcia-Rincon D, Sendtner M, Timmusk T et al (2015) The CB(1) cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ 22(10):1618–1629. doi:10.1038/cdd.2015.11
Bortolotto V, Cuccurazzu B, Canonico PL, Grilli M (2014) NF-kB mediated regulation of adult hippocampal neurogenesis: relevance to mood disorders and antidepressant activity. BioMed Res Int 2014:612798. doi:10.1155/2014/612798
Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, Bankston LA (2002) Cysteine regulation of protein function—as exemplified by NMDA-receptor modulation. Trends Neurosci 25(9):474–480
Ho GP, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, Snyder SH (2011) S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron 71(1):131–141. doi:10.1016/j.neuron.2011.05.033
Selvakumar B, Jenkins MA, Hussain NK, Huganir RL, Traynelis SF, Snyder SH (2013) S-nitrosylation of AMPA receptor GluA1 regulates phosphorylation, single-channel conductance, and endocytosis. Proc Natl Acad Sci U S A 110(3):1077–1082. doi:10.1073/pnas.1221295110
Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, von Mollard GF, Jahn R (1995) The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol 128(4):637–645
Pongrac JL, Slack PJ, Innis SM (2007) Dietary polyunsaturated fat that is low in (n-3) and high in (n-6) fatty acids alters the SNARE protein complex and nitrosylation in rat hippocampus. J Nutr 137(8):1852–1856
Hess DT, Patterson SI, Smith DS, Skene JH (1993) Neuronal growth cone collapse and inhibition of protein fatty acylation by nitric oxide. Nature 366(6455):562–565. doi:10.1038/366562a0
McClintock SM, Husain MM, Greer TL, Cullum CM (2010) Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology 24(1):9–34. doi:10.1037/a0017336
Eu JP, Sun J, Xu L, Stamler JS, Meissner G (2000) The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell 102(4):499–509
Renganathan M, Cummins TR, Waxman SG (2002) Nitric oxide blocks fast, slow, and persistent Na+ channels in C-type DRG neurons by S-nitrosylation. J Neurophysiol 87(2):761–775
Chen J, Daggett H, de Waard M, Heinemann SH, Hoshi T (2002) Nitric oxide augments voltage-gated P/Q-type Ca2+ channels constituting a putative positive feedback loop. Free Radic Biol Med 32(7):638–649. doi:10.1016/S0891-5849(02)00748-7
Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E (2009) Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis/chronic fatigue syndrome. Neuro Endocrinol Lett 30:715–722
Vecchiet J, Cipollone F, Falasca K, Mezzetti A, Pizzigallo E, Bucciarelli T (2003) Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett 335:151–154
Kennedy G, Spence V, McLaren M, Hill A, Underwood C, Belch J (2005) Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med 39:584–589
Richards R, Roberts T, McGregor N, Dunstan R, Butt H (2000) Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep 5:35–41
Shungu D, Weiduschat N, Murrough J, Mao X, Pillemer S, Dyke J (2012) Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed 25:1073–1087
Maes M (2011) An intriguing and hitherto unexplained co-occurrence: depression and chronic fatigue syndrome are manifestations of shared inflammatory, oxidative and nitrosative (IO&NS) pathways. Prog Neuropsychopharmacol Biol Psychiatry 35(3):784–794. doi:10.1016/j.pnpbp.2010.06.023
Manuel y Keenoy B, Moorkens G, Vertommen J, De Leeuw I (2001) Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci 68:2037–2049
Manuel y Keenoy B, Moorkens G, Vertommen J, Noe M, Neve J, De Leeuw I (2000) Magnesium status and parameters of the oxidant-antioxidant balance in patients with chronic fatigue: effects of supplementation with magnesium. J Am Coll Nutr 19:374–382
Miwa K, Fujita M (2010) Fluctuation of serum vitamin E (alpha-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Vessels 25:319–323
Maes M, Mihaylova I, Kubera M, Bosmans E (2007) Not in the mind but in the cell: increased production of cyclo-oxygenase-2 and inducible NO synthase in chronic fatigue syndrome. Neuro Endocrinol Lett 28(4):463–469
Fulle S, Pietrangelo T, Mancinelli R, Saggini R, Fano G (2007) Specific correlations between muscle oxidative stress and chronic fatigue syndrome: a working hypothesis. J Muscle Res Cell Motil 28:355–362
Nunes S, Vargas H, Prado E, Barbosa D, de Melo L, Moylan S (2013) The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neurosci Biobehav Rev 37:1336–1345
Rawdin BJ, Mellon SH, Dhabhar FS, Epel ES, Puterman E, Su Y, Burke HM, Reus VI et al (2013) Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav Immun 31:143–152. doi:10.1016/j.bbi.2012.11.011
Chung CP, Schmidt D, Stein CM, Morrow JD, Salomon RM (2013) Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res 206(2–3):213–216. doi:10.1016/j.psychres.2012.10.018
Leonard B, Maes M (2012) Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 36:764–785
Michel TM, Pulschen D, Thome J (2012) The role of oxidative stress in depressive disorders. Curr Pharm Des 18(36):5890–5899
Yager S, Forlenza M, Miller G (2010) Depression and oxidative damage to lipids. Psychoneuroendocrinology 35:1356–1362
Forlenza M, Miller G (2006) Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med 68:1–7
Tobe E (2013) Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr Dis Treat 9:567–573
Anglin R, Rosebush P, Mazurek M, Tarnopolsky M, Noseworthy M (2011) The psychiatric manifestations of mitochondrial cytopathies: a clinical and MR spectroscopy investigation. Mitochondrion 11:639–640
Moylan S, Berk M, Dean O, Samuni Y, Williams L, O’Neil A (2014) Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev 45C:46–62
Morris G, Anderson G, Galecki P, Berk M, Maes M (2013) A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med 11:64
Acknowledgments
The authors would like to express their thanks to Victoria Storey for her invaluable secretarial services.
Authors’ Contributions
GM and MM participated in the design of this review, while all the other authors helped to draft the paper. All authors read and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Funding
No specific funding was obtained for this specific review. MB is supported by a NHMRC Senior Principal Research Fellowship 1059660.
Rights and permissions
About this article
Cite this article
Morris, G., Berk, M., Klein, H. et al. Nitrosative Stress, Hypernitrosylation, and Autoimmune Responses to Nitrosylated Proteins: New Pathways in Neuroprogressive Disorders Including Depression and Chronic Fatigue Syndrome. Mol Neurobiol 54, 4271–4291 (2017). https://doi.org/10.1007/s12035-016-9975-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9975-2