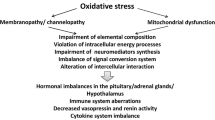
Abstract
Patients who present with severe intractable apparently idiopathic fatigue accompanied by profound physical and or cognitive disability present a significant therapeutic challenge. The effect of psychological counseling is limited, with significant but very slight improvements in psychometric measures of fatigue and disability but no improvement on scientific measures of physical impairment compared to controls. Similarly, exercise regimes either produce significant, but practically unimportant, benefit or provoke symptom exacerbation. Many such patients are afforded the exclusionary, non-specific diagnosis of chronic fatigue syndrome if rudimentary testing fails to discover the cause of their symptoms. More sophisticated investigations often reveal the presence of a range of pathogens capable of establishing life-long infections with sophisticated immune evasion strategies, including Parvoviruses, HHV6, variants of Epstein-Barr, Cytomegalovirus, Mycoplasma, and Borrelia burgdorferi. Other patients have a history of chronic fungal or other biotoxin exposure. Herein, we explain the epigenetic factors that may render such individuals susceptible to the chronic pathology induced by such agents, how such agents induce pathology, and, indeed, how such pathology can persist and even amplify even when infections have cleared or when biotoxin exposure has ceased. The presence of active, reactivated, or even latent Herpes virus could be a potential source of intractable fatigue accompanied by profound physical and or cognitive disability in some patients, and the same may be true of persistent Parvovirus B12 and mycoplasma infection. A history of chronic mold exposure is a feasible explanation for such symptoms, as is the presence of B. burgdorferi. The complex tropism, life cycles, genetic variability, and low titer of many of these pathogens makes their detection in blood a challenge. Examination of lymphoid tissue or CSF in such circumstances may be warranted.

Similar content being viewed by others
Abbreviations
- CFS:
-
Chronic fatigue syndrome
- TNFα:
-
Tumor necrosis factor
- IL:
-
Interleukin
- NF-ΚB:
-
Nuclear factor-ΚB
- IFN:
-
Interferons
- TLR:
-
Toll-like receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- DAMPs:
-
Damage-associated molecular patterns
- MAPK:
-
Mitogen-activated protein kinase
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- 4-HNE:
-
4-Hydroxynonenal
- MDA:
-
Malondialdehyde
- EBV:
-
Epstein-Barr virus
- CAEBV:
-
Chronic activated Epstein-Barr virus syndrome
- ME:
-
Myalgic encephalomyelitis
- CEBVS:
-
Chronic EBV syndrome
- MS:
-
Multiple sclerosis
- MSRV:
-
MS retrovirus
- HHV:
-
Human herpes virus
- Th2:
-
T helper 2
- DCs:
-
Dendritic cells
- Bcl2:
-
B cell lymphoma 2
- BAX:
-
Bcl-2-associated X protein
- PCR:
-
Polymerase chain reaction
- NS1:
-
Nonstructural protein
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- TGF-β1:
-
Transforming growth factor β1
- MRI:
-
Magnetic resonance imaging
- HCMV:
-
Human cytomegalovirus
- Nrf2:
-
Nuclear factor erythroid 2 [NF-E2]-related factor 2
- mTOR:
-
Mammalian target of rapamycin protein
- Bf:
-
Borrelia burgdorferi
- PG:
-
Prostaglandin
- ERK:
-
Extracellular signal-regulated kinase
- COX-2:
-
Cyclooxygenase 2
- SC:
-
Stachybotrys chartarum
- NADH:
-
Reduced nicotinamide adenine dinucleotide
- JNK:
-
c-Jun N-terminal kinase
- FoxP3:
-
Forkhead box P3
- DON:
-
Vomitoxin or deoxynivalenol
- STAT3:
-
Signal transducer and activator of transcription 3
- iNOS:
-
Inducible nitric oxide synthase
References
White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, Baber HL, Burgess M, Clark LV, Cox DL, Bavinton J, Angus BJ, Murphy G, Murphy M, O’Dowd H, Wilks D, McCrone P, Chalder T, Sharpe M, PACE trial management group (2011) Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomized trial. Lancet 377:823–836
Núñez M, Fernández-Solà J, Nuñez E, Fernández-Huerta JM, Godás-Sieso T, Gomez-Gil E (2011) Health-related quality of life in patients with chronic fatigue syndrome: group cognitive behavioural therapy and graded exercise versus usual treatment. A randomised controlled trial with 1year of follow-up. Clin Rheumatol 30:381–389
Morris G, Maes M (2013) Case definitions and diagnostic criteria for Myalgic Encephalomyelitis and Chronic fatigue Syndrome: from clinical-consensus to evidence-based case definitions. Neuro Endocrinol Lett 34:185–199
Morris G, Maes M (2013) Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med 11:205
Morris G, Maes M (2013) A neuro-immune model of myalgic encephalomyelitis/chronic fatigue syndrome. Metab Brain Dis 28:523–540
Hilgers A, Frank J (1994) Chronic fatigue syndrome: immune dysfunction, role of pathogens and toxic agents and neurological and cardial changes. Wien Med Wochenschr 144:399–406
Hilgers A, Krueger GR, Lembke U, Ramon A (1991) Postinfectious chronic fatigue syndrome: case history of thirty-five patients in Germany. In Vivo 5:201–205
Gow JW, Hagan S, Herzyk P, Cannon C, Behan PO, Chaudhuri A (2009) A gene signature for post-infectious chronic fatigue syndrome. BMC Med Genet 2:38
Carlo-Stella N, Badulli C, De Silvestri A, Bazzichi L, Martinetti M, Lorusso L, Bombardieri S, Salvaneschi L, Cuccia M (2006) A first study of cytokine genomic polymorphisms in CFS: positive association of TNF-857 and IFNgamma 874 rare alleles. Clin Exp Rheumatol 24:179–182
Shimosako N, Kerr JR (2014) Use of single-nucleotide polymorphisms (SNPs) to distinguish gene expression subtypes of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Clin Pathol 67(12):1078–1083
Lee KA, Gay CL, Lerdal A, Pullinger CR, Aouizerat BE (2014) Cytokine polymorphisms are associated with fatigue in adults living with HIV/AIDS. Brain Behav Immun 40:95–103
Turvey SE, Hawn TR (2006) Towards subtlety: understanding the role of Toll-like receptor signaling in susceptibility to human infections. Clin Immunol 120:1–9
Misch EA, Hawn TR (2008) Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 114:347–360
Vollmer-Conna U, Piraino B, Cameron B, Davenport T, Hickie I, Wakefield D, Lloyd AE, Dubbo Infection Outcomes Study Group (2008) Cytokine polymorphisms have a synergistic effect on severity of the acute sickness response to infection. Clin Infect Dis 47:1418–1425
Helbig K, Harris R, Ayres J, Dunckley H, Lloyd A, Robson J, Marmion B (2005) Immune response genes in the post-Q-fever fatigue syndrome, Q fever endocarditis and uncomplicated acute primary Q fever. QJM 98:565–574
Helbig K, Heatley S, Harris R, Mullighan C, Bardy P, Marmion B (2003) Variation in immune response genes and chronic Q fever. Concepts: preliminary test with post-Q fever fatigue syndrome. Genes Immun 4:82–85
Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon S, Reeves WC, Lloyd A, Dubbo Infection Outcomes Study Group (2006) Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 333:575
Vollmer-Conna U, Fazou C, Cameron B, Li H, Brennan C, Luck L, Davenport T, Wakefield D, Hickie I, Lloyd A (2004) Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol Med 34:1289–1297
Honstettre A, Imbert G, Ghigo E, Gouriet F, Capo C, Raoult D, Mege J (2003) Dysregulation of cytokines in acute Q fever: role of interleukin-10 and tumor necrosis factor in chronic evolution of Q fever. J Infect Dis 187:956–962
Morris G, Maes M (2014) Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis 29:19–36
Cunningham C (2013) Microglia and neurodegeneration: the role of systemic inflammation. Glia 61:71–90
Lucas K, Maes M (2013) Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol 48:190–204
Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, Álvarez-Vallina L, Sanz L (2014) Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signalingpathway and proinflammatory response in human pericytes. J Biol Chem 289:2457–2468
Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung GH, Yoo BC, Cho JY (2014) Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat Inflamm 2014:352371
Uchida K (2013) Redox-derived damage-associated molecular patterns: ligand function of lipid peroxidation adducts. Redox Biol 1:94–96
Moghaddam AE, Gartlan KH, Kong L, Sattentau QJ (2011) Reactive carbonyls are a major Th2-inducing damage-associated molecular pattern generated by oxidative stress. J Immunol 187:1626–1633
Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO (2013) Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 258:591–596, discussion 596–8
Mathew A, Lindsley TA, Sheridan A, Bhoiwala DL, Hushmendy SF, Yager EJ, Ruggiero EA, Crawford DR (2012) Degraded mitochondrial DNA is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J Alzheimers Dis 30:617–627
Maes M, Twisk FN (2010) Chronic fatigue syndrome: Harvey and Wessely’s (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Med 8:35
Liang Y, Liu J, Feng Z (2013) The regulation of cellular metabolism by tumor suppressor p53. Cell Biosci 3:9
Morris G, Maes M (2012) Increased nuclear factor-κB and loss of p53 are key mechanisms in Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med Hypotheses 79:607–613
Straus SE, Tosato G, Armstrong G, Lawley T, Preble OT, Henle W, Davey R, Pearson G, Epstein J, Brus I, Blaese RM (1985) Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med 102:7–16
Tobi M, Morag A, Ravid Z, Chowers I, Feldman-Weiss V, Michaeli Y, Ben-Chetrit E, Shalit M, Knobler H (1982) Prolonged atypical illness associated with serological evidence of persistent Epstein-Barr virus infection. Lancet 1:61–64
Okano M, Matsumoto S, Osato T, Sakiyama Y, Thiele GM, Purtilo DT (1991) Severe chronic active Epstein-Barr virus infection syndrome. Clin Microbiol Rev 4:129–135
Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, Imai S, Ohga S, Kanegane H, Tsuchiya S, Morio T, Mori M, Yokota S, Imashuku S (2005) Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol 80:64–69
Kimura H, Hoshino Y, Hara S, Sugaya N, Kawada J, Shibata Y, Kojima S, Nagasaka T, Kuzushima K, Morishima T (2005) Differences between T cell-type and natural killer cell-type chronic active Epstein-Barr virus infection. J Infect Dis 191:531–539
Okano M (2000) Haematological associations of Epstein-Barr virus infection. Baillieres Best Pract Res Clin Haematol 13:199–214
Okano M (2011) Features of chronic active Epstein-Barr virus infection and related human diseases. Open Hematol J 5:1–3
Thiele GM, Purtilo DT, Okano M (1991) Differential diagnosis of chronic fatigue syndrome: an update. Infect Med 8:45–51
Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, Jones JF, Dubois RE, Cunningham-Rundles C, Pahwa S et al (1988) Chronic fatigue syndrome: a working case definition. Ann Intern Med 108:387–389
Buchwald D, Goldenberg DL, Sullivan JL, Komaroff AL (1987) The “chronic, active Epstein-Barr virus infection” syndrome and primary fibromyalgia. Arthritis Rheum 30:1132–1136
Hotchin NA, Read R, Smith DG, Crawford DH (1989) Active Epstein-Barr virus infection in post-viral fatigue syndrome. J Infect 18:143–150
Soto NE, Straus SE (2000) Chronic fatigue syndrome and herpesviruses: the fading evidence. Herpes 7:46–50
Swanink CM, van der Meer JW, Vercoulen JH, Bleijenberg G, Fennis JF, Galama JM (1995) Epstein-Barr virus (EBV) and the chronic fatigue syndrome: normal virus load in blood and normal immunologic reactivity in the EBV regression assay. Clin Infect Dis 20:1390–1392
Aydin GB, Akyuz C, Talim B, Evans SE, Sahin S, Sari N, Tabanlioglu D, Ozen S, Cağlar M, Büyükpamukçu M (2007) Extranodal type T/NK-cell lymphoma with an atypical clinical presentation. Pediatr Hematol Oncol 24(4):291–299
Sonke GS, Ludwig I, van Oosten H, Baars JW, Meijer E, Kater AP, de Jong D (2008) Poor outcomes of chronic active Epstein-Barr virus infection and hemophagocytic lymphohistiocytosis in non-Japanese adult patients. Clin Infect Dis 47:105–108
Straus SE (1988) The chronic mononucleosis syndrome. J Infect Dis 157:405–412
Fujieda M, Wakiguchi H, Hisakawa H, Kubota H, Kurashige T (1991) Defective activity of Epstein-Barr virus (EBV) specific cytotoxic T lymphocytes in children with chronic active EBV infection and in their parents. Acta Paediatr Jpn 35:394–399
Sugaya N, Kimura H, Hara S, Hoshino Y, Kojima S, Morishima T, Tsurumi T, Kuzushima K (2004) Quantitative analysis of Epstein-Barr virus (EBV)-specific CD8+ T cells in patients with chronic active EBV infection. J Infect Dis 190:985–988
Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, Gottschalk S, Bollard CM, Rao VK, Marques A, Burbelo PD, Turk SP, Fulton R, Wayne AS, Little RF, Cairo MS, El-Mallawany NK, Fowler D, Sportes C, Bishop MR, Wilson W, Straus SE (2011) Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood 117:5835–5849
Macsween KF, Crawford DH (2003) Epstein-Barr virus-recent advances. Lancet Infect Dis 3:131–140
Kimura H, Morita M, Yabuta Y, Kuzushima K, Kato K, Kojima S, Matsuyama T, Morishima T (1999) Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J Clin Microbiol 37:132–136
Maeda A, Wakiguchi H, Yokoyama W, Hisakawa H, Tomoda T, Kurashige T (1999) Persistently high Epstein-Barr virus (EBV) loads in peripheral blood lymphocytes from patients with chronic active EBV infection. J Infect Dis 179:1012–1015
Patel S, Zuckerman M, Smith M (2003) Real-time quantitative PCR of Epstein-Barr virus BZLF1 DNA using the Light Cycler. J Virol Methods 109:227–233
Kamranvar SA, Masucci MG (2011) The Epstein-Barr virus nuclear antigen-1 promotes telomere dysfunction via induction of oxidative stress. Leukemia 25:1017–1025
Gruhne B, Sompallae R, Marescotti D, Kamranvar SA, Gastaldello S, Masucci MG (2009) The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc Natl Acad Sci U S A 106:2313–2318
Wiedmer A, Wang P, Zhou J, Rennekamp AJ, Tiranti V, Zeviani M, Lieberman PM (2008) Epstein-Barr virus immediate-early protein Zta co-opts mitochondrial single-stranded DNA binding protein to promote viral and inhibit mitochondrial DNA replication. J Virol 82:4647–4655
LaJeunesse DR, Brooks K, Adamson AL (2005) Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 alter mitochondrial morphology during lytic replication. Biochem Biophys Res Commun 333:438–442
Sato Y, Kamura T, Shirata N, Murata T, Kudoh A, Iwahori S, Nakayama S, Isomura H, Nishiyama Y, Tsurumi T (2009) Degradation of phosphorylated p53 by viral protein-ECS E3 ligase complex. PLoS Pathog 5, e1000530
Liu MT, Chang YT, Chen SC, Chuang YC, Chen YR, Lin CS, Chen JY (2005) Epstein-Barr virus latent membrane protein 1 represses p53-mediated DNA repair and transcriptional activity. Oncogene 24:2635–2646
Mauser A, Saito S, Appella E, Anderson CW, Seaman WT, Kenney S (2002) The Epstein-Barr virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms. J Virol 76:12503–12512
Volpi A (2004) Epstein-Barr virus and human herpesvirus type 8 infections of the central nervous system. Herpes Suppl 2:120A–127A
Fujimoto H, Asaoka K, Imaizumi T, Ayabe M, Shoji H, Kaji M (2003) Epstein-Barr virus infections of the central nervous system. Intern Med 42:33–40
Tzartos JS, Khan G, Vossenkamper A, Cruz-Sadaba M, Lonardi S, Sefia E, Meager A, Elia A, Middeldorp JM, Clemens M, Farrell PJ, Giovannoni G, Meier UC (2012) Association of innate immune activation with latent Epstein-Barr virus in active MS lesions. Neurology 78:15–23
García-Montojo M, de la Hera B, Varadé J, de la Encarnación A, Camacho I, Domínguez-Mozo M, Árias-Leal A, García-Martínez A, Casanova I, Izquierdo G, Lucas M, Fedetz M, Alcina A, Arroyo R, Matesanz F, Urcelay E, Alvarez-Lafuente R (2014) HERV-W polymorphism in chromosome X is associated with multiple sclerosis risk and with differential expression of MSRV. Retrovirology 11:2
Kremer D, Schichel T, Förster M, Tzekova N, Bernard C, van der Valk P, van Horssen J, Hartung HP, Perron H, Küry P (2013) Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann Neurol 74:721–732
Mameli G, Poddighe L, Mei A, Uleri E, Sotgiu S, Serra C, Manetti R, Dolei A (2012) Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS One 7, e44991
Kasztelewicz B, Jankowska I, Pawłowska J, Teisseyre J, Dzierżanowska-Fangrat K (2012) The impact of cytokine gene polymorphisms on Epstein-Barr virus infection outcome in pediatric liver transplant recipients. J Clin Virol 55:226–232
Hurme M, Helminen M (1998) Polymorphism of the IL-1 gene complex in Epstein-Barr virus seronegative and seropositive adult blood donors. Scand J Immunol 48:219–222
Binkley PF, Cooke GE, Lesinski A, Taylor M, Chen M, Laskowski B, Waldman WJ, Ariza ME, Williams MV Jr, Knight DA, Glaser R (2013) Evidence for the role of Epstein Barr Virus infections in the pathogenesis of acute coronary events. PLoS One 8, e54008
Agut H (2011) Deciphering the clinical impact of acute human herpesvirus 6 (HHV-6) infections. J Clin Virol 52:164–171
Yao K, Crawford JR, Komaroff AL, Ablashi DV, Jacobson S (2010) Review part 2: human herpesvirus-6 in central nervous system diseases. J Med Virol 82:1669–1678
Birnbaum TCS, Padovan B, Sporer B, Rupprecht TA, Ausserer H, Jaeger G, Pfister HW (2005) Severe meningoencephalitis caused by human herpesvirus 6 type B in an immunocompetent woman treated with ganciclovir. Clin Infect Dis 40:887–889
Isaacson E, Glaser CA, Forghani B, Amad Z, Wallace M, Armstrong RW, Exner MM, Schmid S (2005) Evidence of human herpesvirus 6 infection in 4 immunocompetent patients with encephalitis. Clin Infect Dis 40:890–893
Tavakoli NP, Nattanmai S, Hull R, Fusco H, Dzigua L, Wang H, Dupuis M (2007) Detection and typing of human herpesvirus 6 by molecular methods in specimens from patients diagnosed with encephalitis or meningitis. J Clin Microbiol 45:3972–3978
Fox RI, Saito I, Chan EK, Josephs S, Salahuddin SZ, Ahlashi DV, Staal FW, Gallo R, Pei-Ping H, Le CS (1989) Viral genomes in lymphomas of patients with Sjögren’s syndrome. J Autoimmun 2:449–455
Ranger-Rogez S, Vidal E, Liozon F, Denis F (1994) Primary Sjögren’s syndrome and antibodies to human herpesvirus type 6. Clin Infect Dis 19:1159–1160
Alvarez-Lafuente R, Fernández-Gutiérrez B, de Miguel S, Jover JA, Rollin R, Loza E, Clemente D, Lamas JR (2005) Potential relationship between herpes viruses and rheumatoid arthritis: analysis with quantitative real time polymerase chain reaction. Ann Rheum Dis 64:1357–1359
Broccolo F, Drago F, Paolino S, Cassina G, Gatto F, Fusetti L, Matteoli B, Zaccaria E, Parodi A, Lusso P, Ceccherini-Nelli L, Malnati MS (2009) Reactivation of human herpesvirus 6 (HHV-6) infection in patients with connective tissue diseases. J Clin Virol 46:43–46
Goodman AD, Mock DJ, Powers JM, Baker JV, Blumberg BM (2003) Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J Infect Dis 187:1365–1376
Akhyani N, Berti R, Brennan MB, Soldan SS, Eaton JM, McFarland HF, Jacobson S (2000) Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis 182:1321–1325
Alvarez-Lafuente R, De las Heras V, Bartolomé M, Picazo JJ, Arroyo R (2004) Relapsing-remitting multiple sclerosis and human herpesvirus 6 active infection. Arch Neurol 61:1523–1527
Watt T, Oberfoell S, Balise R, Lunn MR, Kar AK, Merrihew L, Bhangoo MS, Montoya JG (2012) Response to valganciclovir in chronic fatigue syndrome patients with human herpesvirus 6 and Epstein-Barr virus IgG antibody titers. J Med Virol 84:1967–1974
Komaroff AL (2006) Is human herpesvirus-6 a trigger for chronic fatigue syndrome? J Clin Virol 37:S39–S46
Nicolson GL, Gan R, Haier J (2003) Multiple co-infections (Mycoplasma, Chlamydia, human herpes virus-6) in blood of chronic fatigue syndrome patients: association with signs and symptoms. APMIS 111:557–566
Ablashi DV, Josephs SF, Buchbinder A, Hellman K, Nakamura S, Llana T, Lusso P, Kaplan M, Dahlberg J, Memon S et al (1988) Human B-lymphotropic virus (human herpesvirus-6). J Virol Methods 21:29–48
Patnaik M, Komaroff AL, Conley E, Ojo-Amaize EA, Peter JB (1995) Prevalence of IgM antibodies to human herpesvirus 6 early antigen (p41/38) in patients with chronic fatigue syndrome. J Infect Dis 172:1364–1367
Arena A, Liberto MC, Iannello D, Capozza AB, Focà A (1999) Altered cytokine production after human herpes virus type 6 infection. New Microbiol 22:293–300
Flamand L, Gosselin J, D’Addario M, Hiscott J, Ablashi DV, Gallo RC, Menezes J (1991) Human herpesvirus 6 induces interleukin-1 beta and tumor necrosis factor alpha, but not interleukin-6, in peripheral blood mononuclear cell cultures. J Virol 65:5105–5110
Kikuta H, Nakane A, Lu H, Taguchi Y, Minagawa T, Matsumoto S (1990) Interferon induction by human herpesvirus 6 in human mononuclear cells. J Infect Dis 162:35–38
Prusty BK, Böhme L, Bergmann B, Siegl C, Krause E, Mehlitz A, Rudel T (2012) Imbalanced oxidative stress causes chlamydial persistence during non-productive human herpes virus co-infection. PLoS One 7, e47427
Yeo WM, Isegawa Y, Chow VT (2008) The U95 protein of human herpesvirus 6B interacts with human GRIM-19: silencing of U95 expression reduces viral load and abrogates loss of mitochondrial membrane potential. J Virol 82:1011–1020
Li L, Chi J, Zhou F, Guo D, Wang F, Liu G, Zhang C, Yao K (2010) Human herpesvirus 6A induces apoptosis of HSB-2 cells via a mitochondrion-related caspase pathway. J Biomed Res 24:444–451
Kofod-Olsen E, Møller JM, Schleimann MH, Bundgaard B, Bak RO, Øster B, Mikkelsen JG, Hupp T, Höllsberg P (2013) Inhibition of p53-dependent, but not p53-independent, cell death by U19 protein from human herpesvirus 6B. PLoS One 8, e59223
Schleimann MH, Møller JM, Kofod-Olsen E, Höllsberg P (2009) Direct Repeat 6 from human herpesvirus-6B encodes a nuclear protein that forms a complex with the viral DNA processivity factor p41. PLoS One 4, e7457
Reynaud JM, Horvat B (2013) Human herpesvirus 6 and neuroinflammation. ISRN Virol 2013:834890
Opsahl ML, Kennedy PG (2005) Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain 128:516–527
Donati D, Akhyani N, Fogdell-Hahn A, Cermelli C, Cassiani-Ingoni R, Vortmeyer A, Heiss JD, Cogen P, Gaillard WD, Sato S, Theodore WH, Jacobson S (2003) Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections. Neurology 61:1405–1411
Nordström I, Eriksson K (2012) HHV-6B induces IFN-lambda1 responses in cord plasmacytoid dendritic cells through TLR9. PLoS ONE 7, e38683
Frémont M, Metzger K, Rady H, Hulstaert J, De Meirleir K (2009) Detection of herpesviruses and parvovirus B19 in gastric and intestinal mucosa of chronic fatigue syndrome patients. In Vivo 23:209–213
Dominguez-Mozo MI, Garcia-Montojo M, López-Cavanillas M, De Las Heras V, Garcia-Martinez A, Arias-Leal AM, Casanova I, Urcelay E, Arroyo R, Alvarez-Lafuente R (2014) Toll-like receptor-9 in Spanish multiple sclerosis patients: an association with the gender. Eur J Neurol 21:537–540
Chapenko S, Krumina A, Logina I, Rasa S, Chistjakovs M, Sultanova A, Viksna L, Murovska M (2012) Association of active human herpesvirus-6, -7 and parvovirus b19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Adv Virol 2012:205085
Vinnard C, Barton T, Jerud E, Blumberg E (2009) A report of human herpesvirus 6-associated encephalitis in a solid organ transplant recipient and a review of previously published cases. Liver Transpl 15:1242–1246
Norja P, Hokynar K, Aaltonen LM, Chen R, Ranki A, Partio EK, Kiviluoto O, Davidkin I, Leivo T, Eis-Hübinger AM, Schneider B, Fischer HP, Tolba R, Vapalahti O, Vaheri A, Söderlund-Venermo M, Hedman K (2006) Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc Natl Acad Sci U S A 103:7450–7453
Norja P, Eis-Hübinger AM, Söderlund-Venermo M, Hedman K, Simmonds P (2008) Rapid sequence change and geographical spread of human parvovirus B19: comparison of B19 virus evolution in acute and persistent infections. J Virol 82:6427–6433
Manning A, Willey SJ, Bell JE, Simmonds P (2007) Comparison of tissue distribution, persistence, and molecular epidemiology of parvovirus B19 and novel human parvoviruses PARV4 and human bocavirus. J Infect Dis 195:1345–1352
Servant A, Laperche S, Lallemand F, Marinho V, De Saint Maur G, Meritet JF, Garbarg-Chenon A (2002) Genetic diversity within human erythroviruses: identification of three genotypes. J Virol 76:9124–9134
Shackelton LA, Holmes EC (2006) Phylogenetic evidence for the rapid evolution of human B19 erythrovirus. J Virol 80:3666–3669
Corcioli F, Zakrzewska K, Fanci R, De Giorgi V, Innocenti M, Rotellini M, Di Lollo S, Azzi A (2010) Human parvovirus PARV4 DNA in tissues from adult individuals: a comparison with human parvovirus B19 (B19V). Virol J 7:272
Schneider B, Fryer JF, Reber U, Fischer HP, Tolba RH, Baylis SA, Eis-Hübinger AM (2008) Persistence of novel human parvovirus PARV4 in liver tissue of adults. J Med Virol 80:345–351
Matano S, Kinoshita H, Tanigawa K, Terahata S, Sugimoto T (2003) Acute parvovirus B19 infection mimicking chronic fatigue syndrome. Intern Med 42:903–905
Seishima M, Mizutani Y, Shibuya Y, Arakawa C (2008) Chronic fatigue syndrome after human parvovirus B19 infection without persistent viremia. Dermatology 2164:341–346
Kerr JR, Tyrrell DA (2003) Cytokines in parvovirus B19 infection as an aid to understanding chronic fatigue syndrome. Curr Pain Headache Rep 7:333–341
Kerr JR (2005) Pathogenesis of parvovirus B19 infection: host gene variability, and possible means and effects of virus persistence. J Vet Med B Infect Dis Vet Public Health 52:335–339
Sieben M, Schäfer P, Dinsart C, Galle PR, Moehler M (2013) Activation of the human immune system via toll-like receptors by the oncolytic parvovirus H-1. Int J Cancer 132:2548–2556
Hsu GJ, Tzang BS, Tsai CC, Chiu CC, Huang CY, Hsu TC (2011) Effects of human parvovirus B19 on expression of defensins and Toll-like receptors. Chin J Physiol 54:367–376
Raykov Z, Grekova SP, Hörlein R, Leuchs B, Giese T, Giese NA, Rommelaere J, Zawatzky R, Daeffler L (2013) TLR-9 contributes to the antiviral innate immune sensing of rodent parvoviruses MVMp and H-1PV by normal human immune cells. PLoS One 8, e55086
Duechting A, Tschöpe C, Kaiser H, Lamkemeyer T, Tanaka N, Aberle S, Lang F, Torresi J, Kandolf R, Bock CT (2012) Human parvovirus B19 NS1 protein modulates inflammatory signaling by activation of STAT3/PIAS3 in human endothelial cells. J Virol 82(16):7942–7952
Nykky J, Vuento M, Gilbert L (2014) Role of mitochondria in parvovirus pathology. PLoS One 9, e86124
Nykky J, Tuusa JE, Kirjavainen S, Vuento M, Gilbert L (2010) Mechanisms of cell death in canine parvovirus-infected cells provide intuitive insights to developing nanotools for medicine. Int J Nanomedicine 5:417–428
Chia JK, Jackson B (1996) Myopericarditis due to parvovirus B19 in an adult. Clin Infect Dis 23:200–201
Nakashima A, Tanaka N, Tamai K, Kyuuma M, Ishikawa Y, Sato H, Yoshimori T, Saito S, Sugamura K (2006) Survival of parvovirus B19-infected cells by cellular autophagy. Virology 349:254–263
Bucher Praz C, Dessimoz C, Bally F, Reymond S, Troillet N (2012) Guillain-Barré syndrome associated with primary parvovirus B19 infection in an HIV-1-infected patient. Case Rep Med 2012:140780
Hobbs JA (2007) Parvovirus B19-brain interactions: infection, autoimmunity, or both? J Clin Virol 38:364–365
Douvoyiannis M, Litman N, Goldman DL (2009) Neurologic manifestations associated with parvovirus B19 infection. Clin Infect Dis 48:1713–1723
Endresen GK (2003) Mycoplasma blood infection in chronic fatigue and fibromyalgia syndromes. Rheumatol Int 23:211–215
Nijs J, Nicolson GL, De Becker P, Coomans D, De Meirleir K (2022) High prevalence of Mycoplasma infections among European chronic fatigue syndrome patients. Examination of four Mycoplasma species in blood of chronic fatigue syndrome patients. FEMS Immunol Med Microbiol 34:209–214
Zuo LL, Wu YM, You XX (2009) Mycoplasma lipoproteins and Toll-like receptors. J Zhejiang Univ Sci B 10:67–76
Shimizu T, Kida Y, Kuwano K (2008) Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect Immun 76:270–277
Into T, Kiura K, Yasuda M, Kataoka H, Inoue N, Hasebe A, Takeda K, Akira S, Shibata K (2004) Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-kappa B activation. Cell Microbiol 6:187–199
He J, You X, Zeng Y, Yu M, Zuo L, Wu Y (2009) Mycoplasma genitalium-derived lipid-associated membrane proteins activate NF-kappaB through toll-like receptors 1, 2, and 6 and CD14 in a MyD88-dependent pathway. Clin Vaccine Immunol 16:1750–1757
Rawadi G, Roman-Roman S (1996) Mycoplasma membrane lipoproteins induced proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun 64:637–643
Li S, Li X, Wang Y, Yang J, Chen Z, Shan S (2014) Global secretome characterization of A549 human alveolar epithelial carcinoma cells during Mycoplasma pneumoniae infection. BMC Microbiol 14:27
Xu Y, Li H, Chen W, Yao X, Xing Y, Wang X, Zhong J (2013) Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PLoS ONE 8, e77955
Yang J, Hooper WC, Phillips DJ, Talkington DF (2003) Interleukin-1beta responses to Mycoplasma pneumoniae infection are cell-type specific. Microb Pathog 34:17–25
Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ (2012) The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492:123–127
Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T (2012) Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109:11282–11287
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225
Sun G, Xu X, Wang Y, Shen X, Chen Z, Yang J (2008) Mycoplasma pneumoniae infection induces reactive oxygen species and DNA damage in A549 human lung carcinoma cells. Infect Immun 76:4405–44413
Citti C, Nouvel L, Baranowski E (2010) Phase and antigenic variation in mycoplasmas. Future Microbiol 5:1073–1085
van der Merwe J, Prysliak T, Perez-Casal J (2010) Invasion of bovine peripheral blood mononuclear cells and erythrocytes by Mycoplasma bovis. Infect Immun 78:4570–5478
Grover R, Zhu X, Nieusma T, Jones T, Boero I, MacLeod A, Mark A, Niessen S, Kim HJ, Kong L, Assad-Garcia N, Kwon K, Chesi M, Smider VV, Salomon DR, Jelinek DF, Kyle RA, Pyles RB, Glass JI, Ward AB, Wilson IA, Lerner RA (2014) A structurally distinct human mycoplasma protein that generically blocks antigen-antibody union. Science 343:656–661
Hopfe M, Deenen R, Degrandi D, Kohrer K, Henrich B (2013) Host cell responses to persistent mycoplasmas-different stages in infection of HeLa cells with Mycoplasma hominis. Plos One 8:54219
Vancini R, Benchimol M (2008) Entry and intracellular location of Mycoplasma hominis in Trichomonas vaginalis. Arch Microbiol 1891:7–18
McGowin C, Annan R, Quayle A, Greene S, Ma L, Mancuso MM, Adegboye D, Martin DH (2012) Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun 80:3842–3849
Nicolson G, Nasralla M, Haier J, Nicolson N (1998) Diagnosis and treatment of chronic mycoplasmal infections in fibromyalgia and chronic fatigue syndromes: relationship to Gulf War Illness. Biomed Ther 16:266–271
Rogers M (2011) Mycoplasma and cancer: in search of the link. Oncotarget 2:271
Logunov D, Scheblyakov D, Zubkova O, Shmarov M, Rakovskaya I, Gurova K, Tararova ND, Burdelya LG, Naroditsky BS, Ginzburg AL, Gudkov AV (2008) Mycoplasma infection suppresses p53, activates NF-kappaB and cooperates with oncogenic Ras in rodent fibroblast transformation. Oncogene 27:4521–4531
Christo PP, Silva JS, Werneck IV, Dias SL (2010) Rhombencephalitis possibly caused by Mycoplasma pneumoniae. Arq Neuropsiquiatr 68:656–658
Pellegrini M, O’Brien TJ, Hoy J, Sedal L (1996) Mycoplasma pneumoniae infection associated with an acute brainstem syndrome. Acta Neurol Scand 90:203–206
Urbanek C, Goodison S, Chang M, Porvasnik S, Sakamoto N, Li CZ, Boehlein SK, Rosser CJ (2011) Detection of antibodies directed at M. hyorhinis p37 in the serum of men with newly diagnosed prostate cancer. BMC Cancer 11:233
Bahar M, Ashtari F, Aghaei M, Akbari M, Salari M, Ghalamkari S (2012) Mycoplasma pneumonia seroposivity in Iranian patients with relapsing-remitting multipl sclerosis: a randomized case–control study. J Pak Med Assoc 62:6–8
Witkin S, Bierhals K, Linhares I, Normand N, Dieterle S, Neuer A (2010) Genetic polymorphism in an inflammasome component, cervical mycoplasma detection and female infertility in women undergoing in vitro fertilization. J Reprod Immunol 84:171–175
Griffiths P, Whitley R, Snydman DR, Singh N, Boeckh M (2008) International Herpes Management Forum. Contemporary management of cytomegalovirus infection in transplant recipients: guidelines from an IHMF workshop, 2007. Herpes 15:4–12
Griffiths P (1993) Current management of cytomegalovirus disease. J Med Virol 41:106–111
Kano Y, Shiohara T (2000) Current understanding of cytomegalovirus infection in immunocompetent individuals. J Dermatol Sci 22:196–204
Eddleston M, Peacock S, Juniper M, Warrell D (1997) Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis 24:52–56
Wreghitt T, Teare E, Sule O, Devi R, Rice P (2003) Cytomegalovirus infection in immunocompetent patients. Clin Infect Dis 37:1603–1606
Frascaroli G, Varani S, Mastroianni A, Britton S, Gibellini D, Rossini G, Landini MP, Söderberg-Nauclér C (2006) Dendritic cell function in cytomegalovirus-infected patients with mononucleosis. J Leukoc Biol 79:932–940
Yew K, Carpenter C, Duncan R, Harrison C (2012) Human cytomegalovirus induces TLR4 signaling components in monocytes altering TIRAP, TRAM and downstream interferon-beta and TNF-alpha expression. Plos One 7:44500
Compton T, Kurt-Jones E, Boehme K, Belko J, Latz E, Golenbock D, Finberg R (2003) Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 77:4588–4596
Kijpittayarit S, Eid A, Brown R, Paya C, Razonable R (2007) Relationship between Toll-like receptor 2 polymorphism and cytomegalovirus disease after liver transplantation. Clin Infect Dis 44:1315–1320
Wujcicka W, Wilczy’nski J, Nowakowska D (2014) Alterations in TLRs as new molecular markers of congenital infections with Human cytomegalovirus? Pathog Dis 70:3–16
Jablo’nska A, Paradowska E, Studzi’nska M, Suski P, Nowakowska D, Wiśniewska-Ligier M, Woźniakowska-Gęsicka T, Wilczyński J, Leśnikowski ZJ (2014) Relationship between toll-like receptor 2 Arg677Trp and Arg753Gln and toll-like receptor 4 Asp299Gly polymorphisms and cytomegalovirus infection. Int J Infect Dis 25:11–15
Lee G, Kim B (2013) Mitochondria-targeted apoptosis in human cytomegalovirus-infected cells. J Microbiol Biotechnol 23:1627–1635
Andoniou C, Degli-Esposti M (2006) Insights into the mechanisms of CMV-mediated interference with cellular apoptosis. Immunol Cell Biol 84:99–106
Brune W (2011) Inhibition of programmed cell death by cytomegaloviruses. Virus Res 157:144–150
Tanaka K, Zou J, Takeda K, Ferrans V, Sandford G, Johnson T, Finkel T, Epstein SE (1999) Effects of human cytomegalovirus immediate-early proteins on p53-mediated apoptosis in coronary artery smooth muscle cells. Circulation 99:1656–1659
Goldmacher V, Bartle L, Skaletskaya A, Dionne C, Kedersha N, Vater CA, Han JW, Lutz RJ, Watanabe S, Cahir McFarland ED, Kieff ED, Mocarski ES, Chittenden T (1999) A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci U S A 96:12536–12541
O’Brien V (1998) Viruses and apoptosis. J Gen Virol 79:1833–1845
Roulston A, Marcellus R, Branton P (1999) Viruses and apoptosis. Annu Rev Microbiol 53:577–628
Pleskoff O, Casarosa P, Verneuil L, Ainoun F, Beisser P, Smit M, Leurs R, Schneider P, Michelson S, Ameisen JC (2005) The human cytomegalovirus-encoded chemokine receptor US28 induces caspase-dependent apoptosis. FEBS J 272:4163–4177
Rinaldo C, Carney W, Richter B, Black P, Hirsch MS (1980) Mechanisms of immunosuppression in cytomegaloviral mononucleosis. J Infect Dis 141:488–495
Schrier R, Rice G, Oldstone M (1986) Suppression of natural killer cell activity and T cell proliferation by fresh isolates of human cytomegalovirus. J Infect Dis 153:1084–1091
Michelson S (2004) Consequences of human cytomegalovirus mimicry. Hum Immunol 65:465–475
Spencer J, Lockridge K, Barry P, Lin G, Tsang M, Penfold M, Schall T (2002) Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol 76:1285–1292
Beck K, Meyer-Konig U, Weidmann M, Nern C, Hufert F (2003) Human cytomegalovirus impairs dendritic cell function: a novel mechanism of human cytomegalovirus immune escape. Eur J Immunol 33:1528–1538
Varani S, Frascaroli G, Homman-Loudiyi M, Feld S, Landini M, Soderberg-Naucler C (2005) Human cytomegalovirus inhibits the migration of immature dendritic cells by down-regulating cell-surface CCR1 and CCR5. J Leukoc Biol 77:219–228
Moutaftsi M, Brennan P, Spector S, Tabi Z (2004) Impaired lymphoid chemokine-mediated migration due to a block on the chemokine receptor switch in human cytomegalovirus-infected dendritic cells. J Virol 78:3046–3054
Kaarbo M, Ager-Wick E, Osenbroch P, Kilander A, Skinnes R, Muller F, Eide L (2011) Human cytomegalovirus infection increases mitochondrial biogenesis. Mitochondrion 11:935–945
Zhang A, Williamson C, Wong D, Bullough M, Brown K, Hathout Y, Colberg-Poley A (2011) Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol Cell Proteomics 10:M111.009936
Roumier T, Szabadkai G, Simoni AM, Perfettini JL, Paulau AL, Castedo M, Métivier D, Badley A, Rizzuto R, Kroemer G (2006) HIV-1 protease inhibitors and cytomegalovirus vMIA induce mitochondrial fragmentation without triggering apoptosis. Cell Death Differ 13:348–351
Lee Y, Liu C, Cho W, Kuo C, Cheng W, Huang C, Liu C (2014) Presence of cytomegalovirus DNA in leucocytes is associated with increased oxidative stress and subclinical atherosclerosis in healthy adults. Biomarkers 19(2):109–113
Scholz M, Cinatl J, Gross V, Vogel JU, Blaheta RA, Freisleben HJ, Markus BH, Doerr HW (1996) Impact of oxidative stress on human cytomegalovirus replication and on cytokine-mediated stimulation of endothelial cells. Transplantation 61:1763–1770
Jaganjac M, Matijevic T, Cindric M, Cipak A, Mrakovcic L, Gubisch W, Zarkovic N (2010) Induction of CMV-1 promoter by 4-hydroxy-2-nonenal in human embryonic kidney cells. Acta Biochim Pol 57:179–183
Lee J, Koh K, Kim Y, Ahn J, Kim S (2013) Upregulation of Nrf2 expression by human cytomegalovirus infection protects host cells from oxidative stress. J Gen Virol 94:1658–1668
Tilton C, Clippinger A, Maguire T, Alwine J (2011) Human cytomegalovirus induces multiple means to combat reactive oxygen species. J Virol 85:12585–12593
Savaryn JP, Reitsma JM, Bigley TM, Halligan BD, Qian Z, Yu D, Terhune SS (2013) Human cytomegalovirus pUL29/28 and pUL38 repression of p53-regulated p21CIP1 and caspase 1 promoters during infection. J Virol 87:2463–2474
Chen Z, Knutson E, Wang S, Martinez L, Albrecht T (2007) Stabilization of p53 in human cytomegalovirus-initiated cells is associated with sequestration of HDM2 and decreased p53 ubiquitination. J Biol Chem 282:29284–29295
Alcendor DJ, Charest AM, Zhu WQ, Vigil HE, Knobel SM (2012) Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflammation 9:95
Kossmann T, Morganti-Kossmann MC, Orenstein JM, Britt WJ, Wahl SM, Smith PD (2003) Cytomegalovirus production by infected astrocytes correlates with transforming growth factor-beta release. J Infect Dis 187:534–541
Cheeran M, Hu S, Yager S, Gekker G, Peterson P, Lokensgard J (2001) Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J Neurovirol 7:135–147
Orlikowski D, Porcher R, Sivadon-Tardy V, Quincampoix J, Raphaël JC, Durand Raphaël JC, Durand Gaillard JL, Gault E (2011) Guillain–Barr’e syndrome following primary cytomegalovirus infection: a prospective cohort study. Clin Infect Dis 52:837–844
Steininger C, Seiser A, Gueler N, Puchhammer-Stöckl E, Aberle S, Stanek G, Popow-Kraupp T (2007) Primary cytomegalovirus infection in patients with Guillain-Barr’e syndrome. J Neuroimmunol 183:214–219
Cook C (2007) Cytomegalovirus reactivation in“ immunocompetent” patients: a call for scientific prophylaxis. J Infect Dis 196:1273–1275
Ogawa-Goto K, Ueno T, Oshima K, Yamamoto H, Sasaki J, Fujita K, Sata T, Taniguchi S, Kanda Y, Katano H (2012) Detection of active human cytomegalovirus by the promyelocytic leukemia body assay in cultures of PBMCs from patients undergoing hematopoietic stem cell transplantation. J Med Virol 84:479–486
Chandra A, Keilp J, Fallon B (2013) Correlates of perceived health-related quality of life in post-treatment Lyme Encephalopathy. Psychosomatics 54:552–559
Johnson L, Wilcox S, Mankoff J, Stricker R (2014) Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. Peer J 2, e322
Eikeland R, Mygland Å, Herlofson K, Ljostad U (2013) Risk factors for a non-favorable outcome after treated European neuroborreliosis. Acta Neurol Scand 127:154–160
Hildenbrand P, Craven D, Jones R, Nemeskal P (2009) Lyme neuroborreliosis: manifestations of a rapidly emerging zoonosis. AJNR Am J Neuroradiol 30:1079–1087
Rupprecht T, Koedel U, Fingerle V, Pfister H (2008) The pathogenesis of Lyme neuroborreliosis: from infection to inflammation. Mol Med 14:205–212
Kraiczy P, Skerka C, Kirschfink M, Zipfel P, Brade V (2002) Immune evasion of Borrelia burgdorferi: insufficient killing of the pathogens by complement and antibody. Int J Med Microbiol 291:141–146
Strle K, Drouin E, Shen S, El Khoury J, McHugh G, Ruzic-Sabljic E, Strle F, Steere AC (2009) Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis 200:1936–1943
Sandholm K, Henningsson A, Save S, Bergstrom S, Forsberg P, Jonsson N, Ernerudh J, Ekdahl KN (2014) Early cytokine release in response to live Borrelia burgdorferi Sensu Lato spirochetes is largely complement independent. Plos One 9, e108013
Hirschfeld M, Kirschning C, Schwandner R, Wesche H, Weis J, Wooten R, Weis J (1999) Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol 163:2382–2386
Dennis V, Dixit S, O’Brien S, Alvarez X, Pahar B, Philipp M (2009) Live Borrelia burgdorferi spirochetes elicit inflammatory mediators from human monocytes via the Toll-like receptor signaling pathway. Infect Immun 77:1238–1245
Cervantes J, Dunham-Ems S, La Vake C, Petzke M, Sahay B, Sellati TJ, Radolf JD, Salazar JC (2011) Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-beta. Proc Natl Acad Sci U S A 108:3683–3688
Love A, Schwartz I, Petzke M (2014) Borrelia burgdorferi RNA induces type I and III interferons via Toll-like receptor 7 and contributes to production of NF-$kappa$B-dependent cytokines. Infect Immun 82:2405–2416
Cruz A, Moore M, La Vake C, Eggers C, Salazar J, Radolf J (2008) Phagocytosis of Borrelia burgdorferi, the Lyme disease spirochete, potentiates innate immune activation and induces apoptosis in human monocytes. Infect Immun 76:56–70
Cervantes J, Hawley K, Benjamin S, Weinerman B, Luu S, Salazar J (2014) Phagosomal TLR signaling upon Borrelia burgdorferi infection. Front Cell Infect Microbiol 4:55
Ligor M, Olszowy P, Buszewski B (2012) Application of medical and analytical methods in Lyme borreliosis monitoring. Anal Bioanal Chem 402:2233–2248
Łuczaj W, Moniuszko A, Rusak M, Pancewicz S, Zajkowska J, Skrzydlewska E (2011) Lipid peroxidation products as potential bioindicators of Lyme arthritis. Eur J Clin Microbiol Infect Dis 30:415–422
Ratajczak-Wrona W, Jabłońska E, Pancewicz SA, Zajkowska J, Garley M, Iżycka-Herman A, Sawko Ł (2013) Evaluation of serum levels of nitric oxide and its biomarkers in patients with Lyme borreliosis. Prog Health Sci 3:26–32
Bhattacharjee A, Oeemig J, Kolodziejczyk R, Meri T, Kajander T, Lehtinen MJ, Iwaï H, Jokiranta TS, Goldman A (2013) Structural basis for complement evasion by Lyme disease pathogen Borrelia burgdorferi. J Biol Chem 288:18685–18695
Parthasarathy G, Philipp M (2014) The MEK/ERK pathway is the primary conduit for Borrelia burgdorferi-induced inflammation and P53-mediated apoptosis in oligodendrocytes. Apoptosis 19:76–89
Ramesh G, Borda J, Dufour J, Kaushal D, Ramamoorthy R, Lackner A, Philipp M (2008) Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am J Pathol 173:1415–1427
Ramesh G, Santana-Gould L, Inglis F, England J, Philipp M (2013) The Lyme disease spirochete Borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J Neuroinflammation 10:88
Myers T, Kaushal D, Philipp M (2009) Microglia are mediators of Borrelia burgdorferi–induced apoptosis in SH-SY5Y neuronal cells. PLoS Pathog 5, e1000659
Rasley A, Anguita J, Marriott I (2002) Borrelia burgdorferi induces inflammatory mediator production by murine microglia. J Neuroimmunol 130:22–31
Miklossy J, Kasas S, Zurn A, McCall S, Yu S, McGeer P (2008) Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreliosis. J Neuroinflammation 5:1–18
Henningsson AJ, Christiansson M, Tjernberg I, Löfgren S, Matussek A (2014) Laboratory diagnosis of Lyme neuroborreliosis: a comparison of three CSF anti-Borrelia antibody assays. Eur J Clin Microbiol Infect Dis 33:797–803
Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Soloski MJ, Ecker DJ, Schutzer SE, Aucott JN (2012) Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One 7, e36825
Miklossy J (2011) Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J Neuroinflammation 8:90
Crago BR, Gray MR, Nelson LA, Davis M, Arnold L, Thrasher JD (2003) Psychological, neuropsychological and electrocortical effects of mixed mold exposure. Arch Environ Health 58:452–463
Baldo J, Ahmad L, Ruff R (2002) Neuropsychological performance of patients following mold exposure. Appl Neuropsychol 9:193–202
Kilburn KH (2002) Inhalation of molds and mycotoxins. Eur J Oncol 7:197–202
Hope J (2013) A review of the mechanism of injury and treatment approaches for illness resulting from exposure to water-damaged buildings, mold, and mycotoxins. Sci World J 2013:767482
Brasel TL, Douglas DR, Wilson SC, Straus DC (2005) Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins on particulates smaller than conidia. Appl Environ Microbiol 71:114–122
Brasel TL, Martin JM, Carriker CG, Wilson SC, Straus DC (2005) Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Appl Environ Microbiol 71:7376–7388
Cho S-H, Seo S-C, Schmechel D, Grinshpun SS, Reponen T (2005) Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos Environ 39:5454–5465
Charpin-Kadouch C, Maurel G, Felipo R, Queralt J, Ramadour M, Dumon H, Garans M, Botta A, Charpin D (2006) Mycotoxin identification in moldy dwellings. J Appl Toxicol 26:475–479
Górny RL, Reponen T, Willeke K, Schmechel D, Robine E, Boissier M, Grinshpun SA (2002) Fungal fragments as indoor air biocontaminants. Appl Environ Microbiol 68:3522–3531
Creasia DA, Thurman JD, Jones LJ 3rd, Nealley ML, York CG, Wannemacher RW Jr, Bunner DL (1987) Acute inhalation toxicity of T-2 mycotoxin in mice. Fundam Appl Toxicol 8:230–235
Brasel TL, Campbell AW, Demers RE, Ferguson BS, Fink J, Vojdani A, Wilson SC, Straus DC (2004) Detection of trichothecene mycotoxins in sera from individuals exposed to Stachybotrys chartarum in indoor environments. Arch Environ Health 59:317–323
Rocha O, Ansari K, Doohan FM (2005) Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Addit Contam 22:369–378
Zajtchuk R, Bellamy RF (1997) Textbook of military medicine. Borden Institute, Washington
Karunasena E, Larrañaga MD, Simoni JS, Douglas DR, Straus DC (2010) Building-associated neurological damage modeled in human cells: a mechanism of neurotoxic effects by exposure to mycotoxins in the indoor environment. Mycopathologia 170:377–390
Chung YJ, Yang GH, Islam Z, Pestka JJ (2003) Up-regulation of macrophage inflammatory protein-2 and complement 3A receptor by the trichothecenes deoxynivalenol and satratoxin G. Toxicology 186:51–65
Moon Y, Pestka JJ (2003) Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J Nutr Biochem 14:717–726
Moon Y, Uzarski R, Pestka JJ (2003) Relationship of trichothecene structure to COX-2 induction in the macrophage: selective action of type B (8-keto) trichothecenes. J Toxicol Environ Health A 66:1967–1983
Pestka JJ, Zhou HR, Moon Y, Chung YJ (2004) Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol Lett 153:61–73
Zhou HR, Islam Z, Pestka JJ (2003) Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol Sci 72:130–142
Zhou HR, Jia Q, Pestka JJ (2005) Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck. Toxicol Sci 85:916–926
Edmondson DA, Barrios CS, Brasel TL, Straus DC, Kurup VP, Fink JN (2009) Immune response among patients exposed to molds. Int J Mol Sci 10:5471–5484
Gray MR, Thrasher JD, Crago R, Madison RA, Campbell AW, Vojdani A (2003) Mixed mold exposure: immunological changes in humans with exposure in water damaged buildings. Arch Environ Health 58:410–420
Campbell AW, Thrasher JD, Madison RA, Vojdani A, Gray MR, Johnson A (2003) Neural antigen autoantibodies and neurophysiology abnormalities in patients exposed to moulds in water-damaged buildings. Arch Environ 58:464–474
Sorensen B, Streib JE, Strand M, Make B, Giclas PC, Fleshner M, Jones JF (2003) Complement activation in a model of chronic fatigue syndrome. J Allergy Clin Immunol 112:397–403
Thrasher JD, Gray MR, Kilburn KH, Dennis DP, Yu A (2012) A water-damaged home and health of occupants: a case study. J Environ Public Health 2012:312836
Liu J, Wang Y, Cui J, Xing L, Shen H, Wu S, Lian H, Wang J, Yan X, Zhang X (2012) Ochratoxin A induces oxidative DNA damage and G1 phase arrest in human peripheral blood mononuclear cells in vitro. Toxicol Lett 211:164–171
Doi K, Uetsuka K (2011) Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J Mol Sci 12:5213–5237
Bouslimi A, Ouannes Z, Golli EE, Bouaziz C, Hassen W, Bacha H (2008) Cytotoxicity and oxidative damage in kidney cells exposed to the mycotoxins ochratoxin a and citrinin: individual and combined effects. Toxicol Mech Methods 18:341–349
Islam Z, Amuzie CJ, Harkema JR, Pestka JJ (2007) Neurotoxicity and inflammation in the nasal airways of mice exposed to the macrocyclic trichothecene mycotoxin roridin a: kinetics and potentiation by bacterial lipopolysaccharide coexposure. Toxicol Sci 98:526–541
Jussila J, Komulainen H, Kosma VM, Nevalainen A, Pelkonen J, Hirvonen MR (2002) Spores of Aspergillus versicolor isolated from indoor air of a moisture-damaged building provoke acute inflammation in mouse lungs. Inhal Toxicol 14:1261–1277
Cavin C, Delatour T, Marin-Kuan M, Fenaille F, Holzhäuser D, Guignard G, Bezençon C, Piguet D, Parisod V, Richoz-Payot J, Schilter B (2009) Ochratoxin A-mediated DNA and protein damage: roles of nitrosative and oxidative stresses. Toxicol Sci 110:84–94
Zhang X, Jiang L, Geng C, Cao J, Zhong L (2009) The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 54:513–518
Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EM, Doorn JA, Slikker W (2009) Nitrative and oxidative stress in toxicology and disease. Toxicol Sci 112:4–16
Cremer B, Soja A, Sauer JA, Damm M (2012) Pro-inflammatory effects of ochratoxin A on nasal epithelial cells. Eur Arch Otorhinolaryngol 269:1155–1161
Hoehler D, Marquardt RR, McIntosh AR, Hatch GM (1997) Induction of free radicals in hepatocytes, mitochondria and microsomes of rats by ochratoxin A and its analogs. Biochim Biophys Acta 1357:225–233
Sajan MP, Satav JG, Battacharya RK (1997) Effect of aflatoxin B1 in vitro on rat liver mitochondrial respiratory functions. Indian J Exper Biol 35:1187–1190
Bin-Umer MA, McLaughlin JE, Basu D, McCormick S, Tumer NE (2011) Trichothecene mycotoxins inhibit mitochondrial translation–implication for the mechanism of toxicity. Toxins (Basel) 3:1484–1501
Domijan AM, Abramov AY (2011) Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis–implication to mechanism of cell toxicity. Int J Biochem Cell Biol 43:897–904
Kim HY, Jung YH, Hong K, Jang GC, Seo JH, Kwon JW, Kim BJ, Kim HB, Lee SY, Song DJ, Kim WK, Shim JY, Kang MJ, Kim YJ, Yu HS, Hong SJ (2013) Gene-environment interaction between Toll-like receptor 4 and mold exposure in the development of atopic dermatitis in preschool children. Allergy Asthma Respir Dis 1:129–137
Lanciotti M, Pigullo S, Lanza T, Dufour C, Caviglia I, Castagnola E (2008) Possible role of toll-like receptor 9 polymorphism in chemotherapy-related invasive mold infections in children with hematological malignancies. Pediatr Blood Cancer 50:944
Ramirez-Ortiz ZG, Specht CA, Wang JP, Lee CK, Bartholomeu DC, Gazzinelli RT, Levitz SM (2008) Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect Immun 76:2123–2129
Bhan U, Newstead MJ, Zeng X, Podsaid A, Goswami M, Ballinger MN, Kunkel SL, Standiford TJ (2013) TLR9-dependent IL-23/IL-17 is required for the generation of Stachybotrys chartarum-induced hypersensitivity pneumonitis. J Immunol 190:349–356
Larypoor M, Bayat M, Zuhair MH, Akhavan Sepahy A, Amanlou M (2013) Evaluation of the number of CD4(+) CD25(+) FoxP3(+) Treg cells in normal mice exposed to AFB1 and treated with aged garlic extract. Cell J 15:37–44
Azcona-Olivera JI, Ouyang Y, Murtha J, Chu FS, Pestka JJ (1995) Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribution and protein synthesis inhibition. Toxicol Appl Pharmacol 133:109–120
Pinton P, Oswald IP (2014) Effect of deoxynivalenol and other Type B trichothecenes on the intestine: a review. Toxins (Basel) 6:1615–1643
Cano PM, Seeboth J, Meurens F, Cognie J, Abrami R, Oswald IP, Guzylack-Piriou L (2013) Deoxynivalenol as a new factor in the persistence of intestinal inflammatory diseases: an emerging hypothesis through possible modulation of Th17-mediated response. PLoS One 8, e53647
Pestka JJ, Amuzie CJ (2008) Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: comparison of weanling and adult mice. Food Chem Toxicol 46:2826–2831
Amuzie CJ, Shinozuka J, Pestka JJ (2009) Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol Sci 111:277–287
Maresca M, Yahi N, Younes-Sakr L, Boyron M, Caporiccio B, Fantini J (2008) Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: stimulation of interleukin-8 secretion, potentiation of interleukin-1beta effect and increase in the transepithelial passage of commensal bacteria. Toxicol Appl Pharmacol 228:84–92
Maes M, Ringel K, Kubera M, Anderson G, Morris G, Galecki P, Geffard M (2013) In myalgic encephalomyelitis/chronic fatigue syndrome, increased autoimmune activity against 5-HT is associated with immuno-inflammatory pathways and bacterial translocation. J Affect Disord 150:223–230
Maes M, Mihaylova I, Leunis JC (2007) Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J Affect Disord 99:237–240
Akbari P, Braber S, Gremmels H, Koelink PJ, Verheijden KA, Garssen J, Fink-Gremmels J (2014) Deoxynivalenol: a trigger for intestinal integrity breakdown. FASEB J 28:2414–2429
Pinton P, Nougayrede JP, del Rio JC, Moreno C, Marin DE, Ferrier L, Bracarense AP, Kolf-Clauw M, Oswald IP (2009) The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol Appl Pharmacol 237:41–48
Van De Walle J, Sergent T, Piront N, Toussaint O, Schneider YJ, Larondelle Y (2010) Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol Appl Pharmacol 245:291–298
Pinton P, Braicu C, Nougayrede JP, Laffitte J, Taranu I, Oswald IP (2010) Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J Nutr 140:1956–1962
Mbandi E, Pestka JJ (2006) Deoxynivalenol and satratoxin G potentiate proinflammatory cytokine and macrophage inhibitory protein 2 induction by Listeria and Salmonella in the macrophage. J Food Prot 69:1334–1339
Empting LD (2009) Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure. Toxicol Ind Health 25:577–581
Rea WJ, Didriksen N, Simon TR, Pan Y, Fenyves EJ, Griffiths B (2003) Effects of toxic exposure to molds and mycotoxins in building-related illnesses. Arch Environ Health 58:399–405
Kilburn KH (2009) Neurobehavioral and pulmonary impairment in 105 adults with indoor exposure to molds compared to 100 exposed to chemicals. Toxicol Ind Health 25:681–692
Ross GH, Rea WJ, Johnson AR, Hickey DC, Simon TR (1999) Neurotoxicity in single photon emission computed tomography brain scans of patients reporting chemical sensitivities. Toxicol Ind Health 15:415–420
Shifrin VI, Anderson P (1999) Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J Biol Chem 274:13985–13992
Eriksen GS, Petterson H, Lund H (2004) Comparative cytotoxicity of deoxynivalenol, nivalenol, triacetylated derivatives and de-epoxy metabolites. Food Chem Toxicol 42:619–624
Boyd KE, Fitzpatrick DW, Wilson JR, Wilson LM (1988) Effect of T-2 toxin on brain biogenic monoamines in rats and chickens. Can J Vet Res 52:181–185
Wang J, Fiztpatrick DW, Wilson JR (1998) Effects of the trichothecene mycotoxin T-2 toxin on the neurotransmitters and metabolites in discrete areas of the rat brain. Food Chem Toxicol 36:947–953
Galtier P, Paulin F, Eeckhoutte C, Larrieu G (1989) Comparative effects of T-2 toxin and diacetoxyscirpenol on drug metabolizing enzymes in rat tissues. Food Chem Toxicol 27:215–220
Guerre P, Eeckhoutte C, Burgat V, Galtier P (2000) The effects of T-2 toxin exposure on liver drug metabolizing enzymes in rabbit. Food Addit Contam 17:1019–1026
Chaudhary M, Rao PV (2010) Brain oxidative stress after dermal and subcutaneous exposure of T-2 toxin in mice. Food Chem Toxicol 48:3436–3442
Weidner M, Hüwel S, Ebert F, Schwerdtle T, Galla HJ, Humpf HU (2013) Influence of T-2 and HT-2 toxin on the blood–brain barrier in vitro: new experimental hints for neurotoxic effects. PLoS One 8, e60484
Ravindran J, Agrawal M, Gupta N, Rao PV (2011) Alteration of blood brain barrier permeability by T-2 toxin: Role of MMP-9 and inflammatory cytokines. Toxicology 280:44–52
Andersen B, Nielsen KF, Jarvis BB (2002) Characterization of Stachybotrys from water-damaged buildings based on morphology, growth, and metabolite production. Mycologia 94:392–403
Chung YJ, Zhou HR, Pestka JJ (2003) Transcriptional and posttranscriptional roles for p38 mitogen-activated protein kinase in upregulation of TNF-α expression by deoxynivalenol (vomitoxin). Toxicol Appl Pharmacol 193:188–201
Hope JH, Hope BE (2012) A review of the diagnosis and treatment of Ochratoxin A inhalational exposure associated with human illness and kidney disease including focal segmental glomerulosclerosis. J Environ Public Health 2012:835059
Sava V, Reunova O, Velasquez A, Harbison R, Sanchez-Ramos J (2006) Acute neurotoxic effects of the fungal netabolite ochratoxin-A. Neurotoxicology 27:82–92
Sava V, Reunova O, Velasquez A, Sanchez-Ramos J (2006) Can low level exposure to ochratoxin-A cause parkinsonism? J Neurol Sci 249:68–75
Gautier JC, Holzhaeuser D, Markovic J, Gremaud E, Schilter B, Turesky RJ (2001) Oxidative damage and stress response from ochratoxin exposure in rats. Free Radic Biol Med 30:1089–1098
Aleo MD, Wyatt RD, Schnellmann RG (1991) Mitochondrial dysfunction is an early event in ochratoxin A but not oosporein toxicity to rat renal proximal tubules. Toxicol Appl Pharmacol 107:73–80
Zhang X, Boesch-Saadatmandi C, Lou Y, Wolffram S, Huebbe P, Rimbach G (2009) Ochratoxin A induces apoptosis in neuronal cells. Genes Nutr 4:41–48
Zurich MG, Lengacher S, Braissant O, Monnet-Tschudi F, Pellerin L, Honegger P (2005) Unusual astrocyte reactivity caused by the food mycotoxin ochratoxin A in aggregating rat brain cell cultures. Neuroscience 134:771–782
Hong JT, Lee MK, Park KS, Jung KM, Lee RD, Jung HK, Park KL, Yang KJ, Chung YS (2002) Inhibitory effect of peroxisome proliferator-activated receptor gamma agonist on ochratoxin A-induced cytotoxicity and activation of transcription factors in cultured rat embryonic midbrain cells. J Toxicol Environ Health A 65:407–418
Stockmann-Juvala H, Savolainen K (2008) A review of the toxic effects and mechanisms of action of fumonisin B1. Hum Exp Toxicol 27:799–809
Islam Z, Harkema JR, Pestka JJ (2006) Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ Health Perspect 114:1099–1107
Islam Z, Pestka JJ (2006) LPS priming potentiates and prolongs proinflammatory cytokine response to the trichothecene deoxynivalenol in the mouse. Toxicol Appl Pharmacol 211:53–63
Thrasher JD, Crawley S (2009) The biocontaminants and complexity of damp indoor spaces: more than what meets the eyes. Toxicol Ind Health 25:583–615
Alassane-Kpembi I, Kolf-Clauw M, Gauthier T, Abrami R, Abiola FA, Oswald IP, Puel O (2013) New insights into mycotoxin mixtures: the toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol Appl Pharmacol 272:191–198
Tai JH, Pestka JJ (1988) Synergistic interaction between the trichothecene T-2 toxin and Salmonella typhimurium lipopolysaccharide in C3H/HeN and C3H/HeJ mice. Toxicol Lett 44:191–200
Zhou HR, Harkema JR, Yan D, Pestka JJ (1999) Amplified proinflammatory cytokine expression and toxicity in mice coexposed to lipopolysaccharide and the trichothecene vomitoxin (deoxynivalenol). J Toxicol Environ Health A 57(2):115–136
Islam Z, Pestka JJ (2003) Role of IL-1(beta) in endotoxin potentiation of deoxynivalenol-induced corticosterone response and leukocyte apoptosis in mice. Toxicol Sci 74:93–102
Morris G, Berk M, Galecki P, Maes M (2014) The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs). Mol Neurobiol 49:741–756
Morris G, Anderson G, Galecki P, Berk M, Maes M (2013) A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med 11:64
Calderón-Garcidueñas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, Monroy S, DEL Tizapantzi MR, Carson JL, Villarreal-Calderon A, Rewcastle B (2002) Air pollution and brain damage. Toxicol Pathol 30:373–389
Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, Gómez-Garza G, Barragán-Mejía G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR, Henríquez-Roldán C, Pérez-Guillé B, Torres-Jardón R, Herrit L, Brooks D, Osnaya-Brizuela N, Monroy ME, González-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Solt AC, Engle RW (2008) Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn 68:117–127
Calderón-Garcidueñas L, Franco-Lira M, Mora-Tiscareño A, Medina-Cortina H, Torres-Jardón R, Kavanaugh M (2013) Early Alzheimer’s and Parkinson’s disease pathology in urban children: friend versus foe responses—it is time to face the evidence. Biomed Res Int 2013:161687
Acknowledgments
Conflict of Interest
The authors do not report any conflict of interest.
Contributions
All authors contributed equally to the paper.
Funding
There was no specific funding for this specific study.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, G., Berk, M., Walder, K. et al. The Putative Role of Viruses, Bacteria, and Chronic Fungal Biotoxin Exposure in the Genesis of Intractable Fatigue Accompanied by Cognitive and Physical Disability. Mol Neurobiol 53, 2550–2571 (2016). https://doi.org/10.1007/s12035-015-9262-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9262-7