Abstract
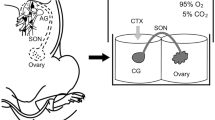
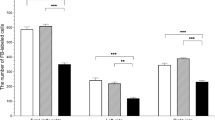
The aim of this study was to determine the influence of estradiol-17β (E2) overdose on the number and distribution of ovarian parasympathetic neurons in the paracervical ganglion (PCG) in adult pigs. To identify the neurons innervating gonads on day 3 of the estrous cycle, the ovaries of both the control and experimental gilts were injected with retrograde neuronal tracer Fast Blue. From next day to the expected day 20 of the second studied cycle, experimental gilts were injected with E2, while control gilts received oil. The PCG were then collected and processed for double-labeling immunofluorescence. Injections of E2 increased the E2 level in the peripheral blood approximately four- to fivefold and reduced the following in the PCG: the total number of Fast Blue-positive neurons; the number of perikarya in the lateral part of the PCG; the numbers of vesicular acetylcholine transporter (VAChT)+/somatostatin+, VAChT+/vasoactive intestinal polypeptide (VIP)+, VAChT+/neuronal isoform of nitric oxide synthase+, VAChT+/VIP−, VAChT+/dopamine β-hydroxylase (DβH)−, VAChT−/VIP−, and VAChT−/DβH− perikarya; and the total number of perikarya expressing estrogen receptors (ERs) subtype α and/or β. In summary, long-term E2 treatment of adult gilts downregulates the population of both cholinergic and ERs expressing the PCG ovary-projecting neurons. Our results suggest that elevated E2 levels occurring during pathological states may regulate gonadal function(s) by affecting ovary-supplying neurons.





Similar content being viewed by others
References
Anesetti G, Lombide P, Chávez-Genaro R (2009) Pre-pubertal estrogen exposure modifies neurotrophin receptor expression in celiac neurons and alters ovarian innervation. Auton Neurosci 145:35–43
Bennett HL, Gustafsson JA, Keast JR (2003) Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton Neurosci 105:90–100
Bódis J, Tinneberg HR, Papenfuss F et al (1993) Cholinergic stimulation of progesterone and estradiol secretion by human granulosa cells cultured in serum-free medium. Gynecol Endocrinol 7:83–87
Böttner M, Wuttke W (2006) Chronic treatment with physiological doses of estradiol affects the GH–IGF-1 axis and fat metabolism in young and middle-aged ovariectomized rats. Biogerontology 7:91–100
Burliński PJ, Burlińska AM, Gonkowski S, Całka J (2012) Resiniferatoxin and tetrodotoxin induced NPY and TH immunoreactivity changes within the paracervical ganglion neurons supplying the urinary bladder. J Mol Neurosci 49(1):62–67. doi:10.1007/s12031-012-9889-z
Cardona-Gómez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM (2001) Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev 37:320–334
Dynarowicz I, Dzięgielewski M (1987) The role of the cholinergic system in the regulation of the blood flow through the reproductive organs of swine during the estrous cycle. Pol Arch Wet 27:69–83 (In Polish)
Ebbert W, Elsaesser F, Bostedt H (1993) Cystic degeneration in porcine ovaries—second communication: concentrations of progesterone, estradiol-17β, and testosterone in cystic fluid and plasma; interpretation of the results. Reprod Domest Anim 28:451–463
Gerendai I, Wiesel O, Tóth E, Boldogköi Z, Hornyák A, Halász B (2005) Occasional transsynaptic viral labeling in the central nervous system from the polycystic ovary induced by estradiol valerate. Microsc Res Tech 5:186–192
Hamilton SA, Garverick HA, Keisler DH et al (1995) Characterization of ovarian follicular cysts and associated endocrine profiles in dairy cows. Biol Reprod 53:890–898
Hiroi H, Osuga Y, Tarumoto Y et al (2002) A case of estrogen-producing Brenner tumor with a stromal component as a potential source for estrogen. Oncology 63:201–204
Jana B, Majewski M (2007) Influence of the peripheral nervous system on ovarian functions. Med Wet 63:1163–1167 (In Polish)
Jana B, Lata M, Bulc M, Całka J (2012) Long term estradiol-17β administration changes population of the dorsal root ganglia neurons innervating the ovary in the sexually mature gilts. Neuropeptides 46:157–165
Jobling P, Lim R (2008) Anatomical and physiological properties of pelvic ganglion neurons in female mice. Auton Neurosci 140:30–39
Kaur G, Janik J, Isaacson LG, Callahan P (2007) Estrogen regulation of neutrophin expression in sympathetic neurons and vascular targets. Brain Res 1139:6–14
Keast JR (1995a) Visualization and immunohistochemical characterization of sympathetic and parasympathetic neurons in the male rat major pelvic ganglion. Neuroscience 66:655–662
Keast JR (1995b) Pelvic ganglia. In: McLachlan EM (ed) Autonomic ganglia. Harwood, Luxembourg, pp 445–479
Keast JR, Saunders RJ (1998) Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs of the male rat. Neuroscience 85:543–556
Kengaku K, Tanaka T, Kamomae H (2007) Changes in the peripheral concentrations of inhibin, follicle-stimulating hormone, luteinizing hormone, progesterone and estradiol-17beta during turnover of cystic follicles in dairy cows with spontaneous follicular cysts. J Reprod Dev 53:987–993
Koszykowska M, Całka J, Szwajca P, Jana B (2011a) Long-term estradiol-17β administration decreases the number of neurons in the caudal mesenteric ganglion innervating the ovary in sexually mature gilts. J Reprod Dev 57:62–71
Koszykowska M, Całka J, Gańko M, Jana B (2011b) Long-term estradiol-17β administration reduces population of neurons in the sympathetic chain ganglia supplying the ovary in adult gilts. Exp Mol Pathol 1:353–361
Koszykowska M, Całka JA, Nidzgorska A, Jana B (2012) Exogenous long-term treatment with 17β-oestradiol alters the innervation pattern in pig ovary. Reprod Fertil Dev. doi:10.1071/RD11271 [epub ahead of print]
Kucharski J, Jana B, Zezula-Szpyra A (2002) Effect of the gonadotrophins treatment on morphological alterations in ovary and peripheral plasma concentrations of steroid hormones in gilts. Pol J Vet Sci 5:7–15
Łakomy M, Kaleczyc J, Całka J (1986) The effect of oestradiolum benzoicum and progesterone on AChE activity in the nerves of the female reproductive system of immature pigs. Gegenbaurs Morphol Jahrb 132:333–348
Liuzzi FJ, Scoville SA, Bufton SM (1999) Long-term estrogen replacement coordinately decreases trkA and beta-PPT mRNA levels in dorsal root ganglion neurons. Exp Neurol 155:260–267
Majewski M (1997) Afferent and efferent innervation of the porcine ovary-sources of origin and chemical coding. Acta Acad Agricult Techn Olst Veterinaria, Supplementum B 24:3–125 (In Polish)
Mitchell BS (1993) Morphology and neurochemistry of the pelvic and paracervical ganglia. Histol Histopathol 8:761–773
Mowa CN, Usip S, Collins J, Storey-Workley M, Hargreaves KM, Papka RE (2003a) The effects of pregnancy and estrogen on the expression of calcitonin gene-related peptide (CGRP) in the uterine cervix, dorsal root ganglia and spinal cord. Peptides 24:1163–1174
Mowa CN, Usip S, Storey-Workley M, Amann R, Papka R (2003b) Substance P in the uterine cervix, dorsal root ganglia and spinal cord during pregnancy and the effect of estrogen on SP synthesis. Peptides 24:761–771
Papka RE, Storey-Workley M (2002) Estrogen receptor-alpha and -beta coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett 319:71–74
Papka RE, Traurig HH, Klenn P (1987) Paracervical ganglia of the female rat: histochemistry and immunohistochemistry of neurons, SIF cells, and nerve terminals. Am J Anat 179:243–257
Papka RE, Srinivasan B, Miller KE, Hayashi S (1997) Localization of estrogen receptor protein and estrogen receptor messenger RNA in peripheral autonomic and sensory neurons. Neuroscience 79:1153–1163
Papka RE, Storey-Workley M, Shughrue PJ et al (2001) Estrogen receptor-alpha and beta-immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res 304:193–214
Papka E, Workley M, Usip S, Mowa CN, Fahrenkrug J (2006) Expression of pituitary adenylate cyclase activating peptide in the uterine cervix, lumbosacral dorsal root ganglia and spinal cord of rats during pregnancy. Peptides 27:743–752
Patrone C, Andersson S, Korhonen L, Lindholm D (1999) Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc Natl Acad Sci USA 96:10905–10910
Podlasz P, Wasowicz K (2008) Neurochemical characteristics of paracervical ganglion in the pig. Vet Med 53:135–146
Puri V, Cui L, Liverman CS, Rob KF et al (2005) Ovarian steroids regulate neuropeptides in the trigeminal ganglion. Neuropeptides 39:409–417
Purves-Tyson TD, Arshi MS, Handelsman DJ, Cheng Y, Keast JR (2007) Androgen and estrogen receptor-mediated mechanisms of testosterone action in male rat pelvic autonomic ganglia. Neuroscience 148:92–104
Sabban EL, Maharjan S, Nostramo R, Serova LI (2010) Divergent effects of estradiol on gene expression of catecholamine biosynthetic enzymes. Physiol Behav 99:163–168
Shinohara Y, Matsumoto A, Hayashi S, Mori T (2000) Prenatal exposure to diethylstilbestrol decreases the number of estrogen receptor alpha-containing neurons innervating the ovary in rat celiac ganglion. Neuroscience 101:779–783
Słomczyńska M (2008) Xenoestrogens: mechanisms of action and some detection studies. Pol J Vet Sci 11:263–269
Sohrabji F, Miranda RC, Toran-Allerand CD (1994) Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci 14:459–471
Swindle MM, Makin A, Herron AJ, Clubb FJ Jr, Frazier KS (2012) Swine as models in biomedical research and toxicology testing. Proc Natl Acad Sci USA 109:16612–16617
Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM (1999) Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res 57:603–615
Tanaka O, Saida TS, Minami R et al (2007) MR findings of the ovarian tumors with hormonal activity, with emphasis on tumors other than sex cord-stromal tumors. Eur J Radiol 62:317–327
Toji S, Watanabe T, Miyagawa I (2008) Effects of long-term estrogen treatment on micturition behavior and the sensory neurons of the urinary bladder in old female rats. Urol Int 81:462–467
Ubuka T, Sakamoto H, Li D, Ukena K, Tsutsui K (2001) Developmental changes in galanin in lumbosacral sympathetic ganglionic neurons innervating the avian uterine oviduct and galanin induction by sex steroids. J Endocrinol 170:357–368
Verma N, Rettenmeier AW, Schmitz-Spanke S (2011) Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 11:776–793
Yang Y, Ozawa H, Lu H, Yuri K, Hayashi S, Nihonyanagi K, Kawata M (1998) Immunohistochemical analysis of sex differences in calcitonin gene-related peptide in the rat dorsal root ganglion, with special references to estrogen and its receptor. Brain Res 791:35–42
Yoshioka K, Iwamura S, Kamomae H (1996) Ultrasonic observations on the turnover of ovarian follicular cysts and associated changes of plasma LH, FSH, progesterone and oestradiol-17 beta in cows. Res Vet Sci 61:240–244
Zhang Y, Tounekti O, Akerman B, Goodyer CG, LeBlanc A (2001) 17-Beta-estradiol induces an inhibitor of active caspases. J Neurosci 21:1–6
Zoubina EV, Smith PG (2002) Distributions of estrogen receptors alpha and beta in sympathetic neurons of female rats: enriched expression by uterine innervation. J Neurobiol 52:14–23
Zoubina EV, Smith PG (2003) Expression of estrogen receptors alpha and beta by sympathetic ganglion neurons projecting to the proximal urethra of female rats. J Urol 169:382–385
Acknowledgments
This work was supported by the State Committee for Scientific Research (grant no. 30801832/1503) and statutory research funds of the Polish Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jana, B., Palus, K., Czarzasta, J. et al. Long-Term Estradiol-17β Administration Changes the Population of Paracervical Ganglion Neurons Supplying the Ovary in Adult Gilts. J Mol Neurosci 50, 424–433 (2013). https://doi.org/10.1007/s12031-012-9950-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9950-y