Abstract
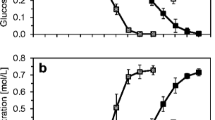
Using a combination of conventional sequential techniques, the batch growth conditions for the production of cell-envelope-associated proteinases have for the first time been studied and optimised in Lactobacillus delbrueckii subsp. lactis 313 (ATCC 7830; LDL 313). Concentrations of inoculum (0.1 < X < 10 % vol/vol), agitation speed (0 < S < 200 rpm), varying incubation temperature (30 < T < 50 °C), starting pH (4.5 < pH < 7) and carbon/nitrogen ratio of production medium (0.2 < r < 5) had an individual effect on proteinase yield (p < 0.01). Optimal conditions for proteinase production included an initial pH of 6.0, 45 °C incubation temperature, 2 % (v/v) inoculum size of OD560 = 1, 150 rpm agitation speed, and growth medium carbon/nitrogen ratio of 1.0. Maximum proteinase activity obtained for whole cells was 0.99 U/ml after 8 h of incubation. The variables studied are very relevant due to their significance in improving the productivity of proteinase synthesis from LDL 313, under process and, likely, economic optimum conditions.









Similar content being viewed by others
References
Kaushik, J. K., Kumar, A., Duary, R. K., Mohanty, A. K., Grover, S., Batish, V. K., et al. (2009). Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS One, 4, e8099.
Gupta, R., Beg, Q. K., Khan, S., & Chauhan, B. (2002). An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Applied Microbiology and Biotechnology, 60, 381–395.
Vesterlund, S., Paltta, J., Laukova, A., Karp, M., & Ouwehand, A. C. (2004). Rapid screening method for the detection of antimicrobial substances. Journal of Microbiological Methods, 57, 23–31.
Tsakalidou, E., Anastasiou, R., Vandenberghe, I., van Beeumen, J., & Kalantzopoulos, G. (1999). Cell-wall-bound proteinase of Lactobacillus delbrueckii subsp. lactis ACA-DC 178: characterization and specificity for β-casein. Applied and Environmental Microbiology, 65, 2035–2040.
Gobbetti, M., Smacchi, E., & Corsetti, A. (1996). The proteolytic system of Lactobacillus sanfrancisco CB1: purification and characterization of a proteinase, a dipeptidase, and an aminopeptidase. Applied and Environmental Microbiology, 62, 3220–3226.
Espeche, T. M. B., Savoy de Giori, G., & Hebert, E. M. (2009). Release of the cell-envelope-associated proteinase of Lactobacillus delbrueckii subspecies lactis CRL 581 is dependent upon pH and temperature. Journal of Agricultural and Food Chemistry, 57, 8607–8611.
Potumarthi, R., Ch, S., & Jetty, A. (2007). Alkaline protease production by submerged fermentation in stirred tank reactor using Bacillus licheniformis NCIM-2042: effect of aeration and agitation regimes. Biochemical Engineering Journal, 34, 185–192.
Rosso, A. M., Ferrarotti, S. A., Krymkiewicz, N., & Nudel, B. C. (2002). Optimisation of batch culture conditions for cyclodextrin glucanotransferase production from Bacillus circulans DF 9R. Microbial Cell Factories, 1, 3.
Danquah, M. K., & Forde, G. M. (2007). Growth medium selection and its economic impact on plasmid DNA production. Journal of Bioscience and Bioengineering, 104, 490–497.
Hebert, E., Mamone, G., Picariello, G., Raya, R., Savoy, G., Ferranti, P., et al. (2008). Characterization of the pattern of αs1-and β-casein breakdown and release of a bioactive peptide by a cell envelope proteinase from Lactobacillus delbrueckii subsp. lactis CRL 581. Applied and Environmental Microbiology, 74, 3682–3689.
Kojic, M., Fira, D., Banina, A., & Topisirovic, L. (1991). Characterization of the cell wall-bound proteinase of Lactobacillus casei HN14. Applied and Environmental Microbiology, 57, 1753–1757.
Khalid, N. M., & Marth, E. H. (1990). Lactobacilli—their enzymes and role in ripening and spoilage of cheese: a review. Journal of Dairy Science, 73, 2669–2684.
Laloi, P., Atlan, D., Blanc, B., Gilbert, C., & Portalier, R. (1991). Cell-wall-associated proteinase of Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397: differential extraction, purification and properties of the enzyme. Applied Microbiology and Biotechnology, 36, 196–204.
Martín-Hernández, M. C., Alting, A. C., & Exterkate, F. A. (1994). Purification and characterization of the mature, membrane-associated cell-envelope proteinase of Lactobacillus helveticus L89. Applied Microbiology and Biotechnology, 40, 828–834.
Valu, J. A. (1965). Survival of Lactobacillus leichmannii in relation to vitamin B12 assays. Applied and Environmental Microbiology, 13, 486–490.
Taranto, M. P., Vera, J. L., Hugenholtz, J., De Valdez, G. F., & Sesma, F. (2003). Lactobacillus reuteri CRL1098 produces Cobalamin. Journal of Bacteriology, 185, 5643–5647.
Batdorj, B., Trinetta, V., Dalgalarrondo, M., Prévost, H., Dousset, X., Ivanova, I., et al. (2007). Isolation, taxonomic identification and hydrogen peroxide production by Lactobacillus delbrueckii subsp. lactis T31, isolated from Mongolian yoghurt: inhibitory activity on food-borne pathogens. Journal of Applied Microbiology, 103, 584–593.
Vogel, R. F., & Ehrmann, M. A. (2008). Sourdough fermentations. In L. Cocolin & D. Ercolini (Eds.), Molecular techniques in the microbial ecology of fermented foods (pp. 119–144). New York: Springer.
Agyei, D., & Danquah, M. K. (2011). Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnology Advances, 29, 272–277.
Zwietering, M. H., Jongenburger, I., Rombouts, F. M., & VAN T Riet, K. (1990). Modeling of the bacterial growth curve. Applied and Environmental Microbiology, 56, 1875–1881.
Exterkate, F. A. (1990). Differences in short peptide-substrate cleavage by two cell-envelope-located serine proteinases of Lactococcus lactis subsp. cremoris are related to secondary binding specificity. Applied Microbiology and Biotechnology, 33, 401–406.
Abusham, R. A., Rahman, R. N., Salleh, A. B., & Basri, M. (2009). Optimization of physical factors affecting the production of thermo-stable organic solvent-tolerant protease from a newly isolated halo tolerant Bacillus subtilis strain Rand. Microbial Cell Factories, 8, 20.
Pessôa de França, F., Marieta de Jesus, A., & Oliveira, F. J. S. (2009). Enhancement of lactic acid fermentation by Lactobacillus delbrueckii ATCC 6949 using sugarcane molasses. Canada Journal of Pure and Applied Science, 3, 774–778.
Koutsoumanis, K. P., & Sofos, J. N. (2005). Effect of inoculum size on the combined temperature, pH and aw limits for growth of Listeria monocytogenes. International Journal of Food Microbiology, 104, 83–91.
Marugg, J., Meijer, W., van Kranenburg, R., Laverman, P., Bruinenberg, P., & de Vos, W. (1995). Medium-dependent regulation of proteinase gene expression in Lactococcus lactis: control of transcription initiation by specific dipeptides. Journal of Bacteriology, 177, 2982–2989.
Mackey, B. M., & Kerridge, A. L. (1988). The effect of incubation temperature and inoculum size on growth of salmonellae in minced beef. International Journal of Food Microbiology, 6, 57–65.
Gupta, S., Abu-Ghannam, N., & Scannell, A. G. M. (2011). Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioproducts Process, 89, 345–355.
Tango, M. S. A., & Ghaly, A. E. (1999). Amelioration of lactic acid production from cheese whey using micro-aeration. Biomass Bioenergetics, 17, 221–238.
Murphy, M. G., & Condon, S. (1984). Comparison of aerobic and anaerobic growth of Lactobacillus plantarum in a glucose medium. Archives of Microbiology, 138, 49–53.
Agyei, D., & Danquah, M. K. (2012). In-depth characterisation of Lactobacillus delbrueckii subsp. lactis 313 for growth and cell-envelope-associated proteinase production. Biochemical Engineering Journal, 64, 61–68.
Axelsson, L. (2004). Lactic acid bacteria: Classification and physiology. In S. Salminen, A. von Wright, & A. Ouwehand (Eds.), Lactic acid bacteria. Microbiological and functional aspects (pp. 1–66). New York: Marcel Dekker.
Kandler, O. (1983). Carbohydrate metabolism in lactic acid bacteria. Anton Leeuw, 49, 209–224.
Hickey, M. W., Hillier, A. J., & Jago, G. R. (1983). Metabolism of pyruvate and citrate in lactobacilli. Australian Journal of Biological Sciences, 36, 487–496.
Sakamoto, M., & Komagata, K. (1996). Aerobic growth of and activities of NADH oxidase and NADH peroxidase in lactic acid bacteria. Journal of Fermentation and Bioengineering, 82, 210–216.
Ibrahim, S. B., Rahman, N. A. A., Mohamad, R., & Rahim, R. A. (2010). Effects of agitation speed, temperature, carbon and nitrogen sources on the growth of recombinant Lactococcus lactis NZ9000 carrying domain 1 of aerolysin gene. African Journal of Biotechnology, 9, 5392–5398.
Sepahy, A.A., and L. Jabalameli. (2011). Effect of culture conditions on the production of an extracellular protease by Bacillus sp. isolated from soil sample of Lavizan Jungle Park. Enzyme Res., 2011 (in press).
Saurabh, S., Jasmine, I., Pritesh, G., & Kumar, S. R. (2007). Enhanced productivity of serine alkaline protease by Bacillus sp. using soybean as substrate. Malaysian Journal of Microbiology, 3, 1–6.
Panesar, P. S., Kennedy, J. F., Knill, C. J., & Kosseva, M. (2010). Production of L(+) lactic acid using Lactobacillus casei from whey. Brazilian Archives of Biology and Technology, 53, 219–226.
Membré, J.-M., Leporq, B., Vialette, M., Mettler, E., Perrier, L., Thuault, D., et al. (2005). Temperature effect on bacterial growth rate: quantitative microbiology approach including cardinal values and variability estimates to perform growth simulations on/in food. International Journal of Food Microbiology, 100, 179–186.
Kumar, C. G., & Takagi, H. (1999). Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnology Advances, 17, 561–594.
Perego, P., Converti, A., & Del Borghi, M. (2003). Effects of temperature, inoculum size and starch hydrolyzate concentration on butanediol production by Bacillus licheniformis. Bioresource Technology, 89, 125–131.
Sampaio, F. C., de Moraes, C. A., De Faveri, D., Perego, P., Converti, A., & Passos, F. M. L. (2006). Influence of temperature and pH on xylitol production from xylose by Debaryomyces hansenii UFV-170. Process Biochemistry, 41, 675–681.
Young, T. W., Wadeson, A., Glover, D. J., Quincey, R. V., Butlin, M. J., & Kamei, E. A. (1996). The extracellular acid protease gene of Yarrowia lipolytica: sequence and pH-regulated transcription. Microbiology, 142, 2913–2921.
Ellaiah, P., Srinivasulu, B., & Adinarayana, K. (2002). A review on microbial alkaline proteases. Anglais, 61, 15.
Das Mohapatra, P. K., Maity, C., Rao, R. S., Pati, B. R., & Mondal, K. C. (2009). Tannase production by Bacillus licheniformis KBR6: optimization of submerged culture conditions by Taguchi DOE methodology. Food Research International, 42, 430–435.
Gilbert, C., Blanc, B., Frot-coutaz, J., Portalier, R., & Atlan, D. (1997). Comparison of cell surface proteinase activities within the Lactobacillus genus. The Journal of Dairy Research, 64, 561–571.
Titgemeyer, F., & Hillen, W. (2002). Global control of sugar metabolism: a Gram-positive solution. Anton Leeuwenhoek, 82, 59–71.
Degeest, B., & De Vuyst, L. (1999). Indication that the Nitrogen source influences both amount and size of exopolysaccharides produced by Streptococcus thermophilus LY03 and modelling of the bacterial growth and exopolysaccharide production in a complex medium. Applied and Environmental Microbiology, 65, 2863–2870.
Mataragas, M., Drosinos, E. H., Tsakalidou, E., & Metaxopoulos, J. (2004). Influence of nutrients on growth and bacteriocin production by Leuconostoc mesenteroides L124 and Lactobacillus curvatus L442. Anton Leeuwenhoek, 85, 191–198.
Singh, R., Kapoor, V., & Kumar, V. (2011). Influence of carbon and nitrogen sources on the α-amylase production by a newly isolated thermophilic Streptomyces sp. MSC702 (MTCC 10772). Asian Journal Biotechnology, 3, 540–553.
Hebert, E. M., Raya, R. R., & De Giori, G. S. (2000). Nutritional requirements and nitrogen-dependent regulation of proteinase activity of Lactobacillus helveticus CRL 1062. Applied and Environmental Microbiology, 66, 5316–5321.
Pastar, I., Tonic, I., Golic, N., Kojic, M., van Kranenburg, R., Kleerebezem, M., et al. (2003). Identification and genetic characterization of a novel proteinase, PrtR, from the human isolate Lactobacillus rhamnosus BGT10. Applied and Environmental Microbiology, 69, 5802–5811.
Tomas, M. S. J., Labanda, E. B. D., de Ruiz Holgado, A. P., Nader-Macias, M. E., et al. (2002). Estimation of vaginal probiotic lactobacilli growth parameters with the application of the Gompertz model. Canadian Journal of Microbiology, 48, 82–92.
Acknowledgement
The authors wish to thank the Monash University Graduate School for providing funding for this study.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 56 kb)
Rights and permissions
About this article
Cite this article
Agyei, D., Potumarthi, R. & Danquah, M.K. Optimisation of Batch Culture Conditions for Cell-Envelope-Associated Proteinase Production from Lactobacillus delbrueckii subsp. lactis ATCC® 7830™. Appl Biochem Biotechnol 168, 1035–1050 (2012). https://doi.org/10.1007/s12010-012-9839-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9839-9