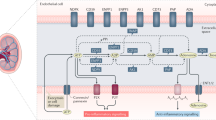
Abstract
Extracellular ATP interacts with purinergic type 2 (P2) receptors and elicits many crucial biological functions. Extracellular ATP is sequentially hydrolyzed to ADP and AMP by the actions of defined nucleotidases, such as CD39, and AMP is converted to adenosine, largely by CD73, an ecto-5′-nucleotidase. Extracellular adenosine interacts with P1 receptors and often opposes the effects of P2 receptor activation. The balance between extracellular ATP and adenosine in the blood and extracellular fluid is regulated chiefly by the activities of CD39 and CD73, which constitute the CD39-adenosinergic axis. In recent years, several studies have shown this axis to play critical roles in transport of water/sodium, tubuloglomerular feedback, renin secretion, ischemia reperfusion injury, renal fibrosis, hypertension, diabetic nephropathy, transplantation, inflammation, and macrophage transformation. Important developments include global and targeted gene knockout and/or transgenic mouse models of CD39 or CD73, biological or small molecule inhibitors, and soluble engineered ectonucleotidases to directly impact the CD39-adenosinergic axis. This review presents a comprehensive picture of the multiple roles of CD39-adenosinergic axis in renal physiology, pathophysiology, and therapeutics. Scientific advances and greater understanding of the role of this axis in the kidney, in both health and illness, will direct development of innovative therapies for renal diseases.







Similar content being viewed by others
References
Burnstock G (2009) Purinergic signaling: past, present and future. Braz J Med Biol Res 42(1):3–8. https://doi.org/10.1590/S0100-879X2008005000037
Burnstock G (2011) Introductory overview of purinergic signalling. Front Biosci 3:896–900
Burnstock G (2012) Purinergic signaling: its unpopular beginning, its acceptance and its exciting future. BioEssays 34(3):218–225. https://doi.org/10.1002/bies.201100130
Burnstock G (2013) Introduction and perspective, historical note. Front Cell Neurosci 7:227. https://doi.org/10.3389/fncel.2013.00227
Burnstock G (2014) The Paton lecture: purinergic signalling: from discovery to current developments. Exp Physiol 99(1):16–34. https://doi.org/10.1113/expphysiol.2013.071951
Burnstock G (2016) Short- and long-term (trophic) purinergic signalling. Philos Trans R Soc B 371(1700):20150422. https://doi.org/10.1098/rstb.2015.0422
Jacobson KA (2009) Introduction to adenosine receptors as therapeutic targets. Handb Exp Pharmacol 193:1–24. https://doi.org/10.1007/978-3-540-89615-9_1
Jacobson KA, Boeynaems JM (2010) P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today 15(13-14):570–578. https://doi.org/10.1016/j.drudis.2010.05.011
Fredholm BB (2010) Adenosine receptors as drug targets. Exp Cell Res 316(4):1284–1288. https://doi.org/10.1038/nrd3955
Samsel M, Dzierzbicka K (2011) Therapeutic potential of adenosine analogues and conjugates. Pharmacol Rep 63(3):601–617. https://doi.org/10.1016/S1734-1140(11)70573-4
Riksen NP, Rongen GA (2012) Targeting adenosine receptors in the development of cardiovascular therapeutics. Expert Rev Clin Pharmacol 5(2):199–218. https://doi.org/10.1586/ecp.12.8
Chen JF, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets – what are the challenges? Nat Rev Drug Discov 12(4):265–286. https://doi.org/10.1038/nrd3955
Kishore BK, Carlson NG, Ecelbarger CM, Kohan DE, Müller CE, Nelson RD, Peti-Peterdi J, Zhang Y (2015) Targeting renal purinergic signalling for the treatment of lithium-induced nephrogenic diabetes insipidus. Acta Physiol (Oxf) 214(2):176–188. https://doi.org/10.1111/apha.12507
Jankowski M (2008) Purinergic regulation of glomerular microvasculature and tubular function. J Physiol Pharmacol 59:121–135
Wildmann SS, King BF (2008) P2X receptors: epithelial ion channels and regulators of salt and water transport. Nephrol Physiol 108(3):60–67. https://doi.org/10.1159/000122028
Vallon V (2008) P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Ren Physiol 294(1):F10–F27. https://doi.org/10.1152/ajprenal.00432.2007
Lu B, Rajakumar SV, Robson SC, Lee EK, Crikis S, d’Apice AJ, Cowan PJ, Dwyer KM (2008) The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation 86(12):1707–1712. https://doi.org/10.1097/TP.0b013e31819022bc
Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE (2009) P2Y2 receptor and water transport in the kidney. Purinergic Signal 5(4):491–499. https://doi.org/10.1007/s11302-009-9151-5
Vallon V, Osswald H (2009) Adenosine receptors and the kidney. Handb Exp Pharmacol 193:443–470. https://doi.org/10.1007/978-3-540-89615-9_15
Prætorius H, Leipziger J (2010) Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72(1):377–393. https://doi.org/10.1146/annurev-physiol-021909-135825
Leipziger J (2011) Luminal nucleotides are tonic inhibitors of renal tubular transport. Curr Opin Nephrol Hypertens 20(5):518–522. https://doi.org/10.1097/MNH.0b013e3283487393
Laubach VE, French BA, Okusa MD (2011) Targeting of adenosine receptors in ischemia-reperfusion injury. Expert Opin Ther Targets 15(1):103–118. https://doi.org/10.1517/14728222.2011.541441
Vallon V, Rieg T (2011) Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Ren Physiol 301(3):F463–F475. https://doi.org/10.1152/ajprenal.00236.2011
Guan Z, Inscho EW (2011) Role of adenosine 5′-triphosphate in regulating renal microvascular function and in hypertension. Hypertension 58(3):333–340. https://doi.org/10.1161/HYPERTENSIONAHA.110.155952
Booth JW, Tam FW, Unwin RJ (2012) P2 purinoceptors: renal pathophysiology and therapeutic potential. Clin Nephrol 78(08):154–163. https://doi.org/10.1161/HYPERTENSIONAHA.110.155952
Roberts V, Lu B, Rajakumar S, Cowan PJ, Dwyer KM (2013) The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal 9(2):135–143. https://doi.org/10.1007/s11302-012-9342-3
Burnstock G, Evans LC, Bailey MA (2014) Purinergic signalling in the kidney in health and disease. Purinergic Signal 10(1):71–101. https://doi.org/10.1007/s11302-013-9400-5
Guan Z, Fellner RC, Van Beusecum J, Inscho EW (2014) P2 receptors in renal autoregulation. Curr Vasc Pharmacol 12(6):818–828. https://doi.org/10.2174/15701611113116660152
Howarth AR, Conway BR, Bailey MA (2015) Vascular inflammatory actions of P2X receptors in renal injury. Auton Neurosci 191:135–140. https://doi.org/10.1016/j.autneu.2015.05.001
Peti-Peterdi J, Kishore BK, Pluznick JL (2015) Regulation of vascular and renal function by metabolite receptors. Annu Rev Physiol 78(1):391–414. https://doi.org/10.1146/annurev-physiol-021115-105403
Van Beusecum J, Inscho EW (2015) Regulation of renal function and blood pressure control by P2 purinoceptrs in the kidney. Curr Opin Pharmacol 21:82–88. https://doi.org/10.1016/j.coph.2015.01.003
Franco M, Bautista-Pérez R, Pérez-Méndez O (2015) Purinergic receptors in tubulointerstitial inflammatory cells: a pathophysiological mechanism of salt-sensitive hypertension. Acta Physiol (Oxf) 214(1):75–87. https://doi.org/10.1111/apha.12471
Solini A, Usuelli V, Florina P (2015) The dark side of extracellular ATP in kidney disease. J Am Soc Nephrol 26(5):1007–1016. https://doi.org/10.1681/ASN.2014070721
Menzies RI, Unwin RJ, Bailey MA (2015) Renal P2 receptors and hypertension. Acta Physiol (Oxf) 213(1):232–241. https://doi.org/10.1111/apha.12412
Menzies RI, Tam FW, Unwin RJ, Bailey MA (2017) Purinergic signaling in kidney disease. Kidney Int 91(2):315–325. https://doi.org/10.1016/j.kint.2016.08.029
Ilatovskaya DV, Palygin O, Staruschenko A (2016) Functional and therapeutic importance of purinergic signaling in polycystic kidney disease. Am J Physiol Ren Physiol 311(6):F1135–F1139. https://doi.org/10.1152/ajprenal.00406.2016
Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM (2011) Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol 61:221–261. https://doi.org/10.1016/B978-0-12-385526-8.00008-4
Lohman AW, Isakson BE (2014) Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett 588(8):1379–1388. https://doi.org/10.1016/j.febslet.2014.02.004
Unwin RJ, Bailey MA, Burnstock G (2003) Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci 18(6):237–241. https://doi.org/10.1152/nips.01436.2003
Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmerman H, Sévigny J, Robson SC (2005) Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Ren Physiol 288(5):F1032–F1043. https://doi.org/10.1152/ajprenal.00108.2004
Vekaria RM, Shirley DG, Sévigny J, Unwin RJ (2006) Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Ren Physiol 290(2):F550–F560. https://doi.org/10.1152/ajprenal.00151.2005
Zhang Y, Robson SC, Morris KL, Heiney KM, Dwyer KM, Kishore BK, Ecelbarger CM (2015) Impaired natriuretic response to high-NaCl diet plus aldosterone infusion in mice overexpressing human CD39, an ectonucleotidase (NTPDase1). Am J Physiol Ren Physiol 308(12):F1398–F1408. https://doi.org/10.1152/ajprenal.00125.2014
Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationship and pathophysiological significance. Purinergic Signal 2(2):409–430. https://doi.org/10.1007/s11302-006-9003-5
Schetinger MR, Morsch VM, Bonan CD, Wyse AT (2007) NTPDase and 5′-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors 31(2):77–98. https://doi.org/10.1002/biof.5520310205
Yegtkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signaling cascade. Biochim Biophys Acta 1783(5):673–694. https://doi.org/10.1016/j.bbamcr.2008.01.024
Kukulski F, Levesque SA, Sévigny J (2011) Impact of ectoenzymes on P2 and P1 receptor signaling. Adv Pharmacol 61(5):263–299. https://doi.org/10.1016/j.bbamcr.2008.01.024
Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8(3):437–502. https://doi.org/10.1007/s11302-012-9309-4
Kishore BK, Zhang Y, Gevorgyan K, Kohan DE, Schiedel AC, Müller CE, Peti-Peterdi J (2013) Cellular localization of adenine receptors in the rat kidney and their functional significance in the inner medullary collecting duct. Am J Physiol Ren Physiol 305(9):F1298–F1305. https://doi.org/10.1152/ajprenal.00254.2013
Thimm D, Schiedel AC, Peti-Peterdi J, Kishore BK, Müller CE (2015) The nucleobase adenine as a signalling molecule in the kidney. Acta Physiol (Oxf) 213(4):808–818. https://doi.org/10.1111/apha.12452
Slominska E, Szolkiewicz M, Smolenski RT, Rutkowski B, Swierczynski J (2002) High plasma adenine concentration in chronic renal failure and its relation to erythrocyte ATP. Nephron 91:286–291. https://doi.org/10.1159/000058406
Knepper MA, Kwon TH, Nielsen S (2015) Molecular physiology of water balance. N Engl J Med 372(14):1349–1358. https://doi.org/10.1056/NEJMra1404726
Shibata S (2017) 30 years of mineralocorticoid receptor: mineralocorticoid receptor and NaCl transport mechanisms in the renal distal nephrol. J Endocrinol 234(1):T35–T47. https://doi.org/10.1530/JOE-16-0669
Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, Fisicaro N, Mysore TB, Kaczmarek E, Cowan PJ, d’Apice AJ (2004) Thromboregulatory manifestations in human CD transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest 113(10):1440–1446. https://doi.org/10.1172/JCI19560
Zhang Y, Morris KL, Sparrow SK, Dwyer KM, Enjyoji K, Robson SC, Kishore BK (2012) Defective renal water handling in transgenic mice over-expressing human CD39/NTPDase1. Am J Physiol Ren Physiol 303(3):F420–F430. https://doi.org/10.1152/ajprenal.00060.2012
Calström M, Wilcox CS, Arendshors WJ (2015) Renal autoregulation in health and disease. Physiol Rev 95(2):405–511. https://doi.org/10.1152/physrev.00042.2012
Ferenbach DA, Bonventre JV (2016) Kidney tubules: intertubular, vascular, and glomerular cross-talk. Curr Opin Nephrol Hypertens 25(3):194–202. https://doi.org/10.1097/MNH0000000000000218
Nishiyama A, Navar LG (2002) ATP mediates tubuloglomerular feedback. Am J Phys Regul Integr Comp Phys 283(1):R273–R275. https://doi.org/10.1152/ajpregu.00071.2002
Bell PD, Komlos P, Zhang ZR (2009) ATP as a mediator of macula densa cell signaling. Purinergic Signal 5(4):461–471. https://doi.org/10.1007/s11302-009-9148-0
Franco M, Bell PD, Navar LG (1989) Effect of adenosine A1 analogue on tubuloglomerular feedback mechanism. Am J Physiol Ren Physiol 257:F231–F236
Brown R, Ollerstam A, Johansson B, Skøtt O, Gebre-Medhin S, Fredholm B, Persson AE (2001) Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Phys Regul Integr Comp Phys 281(5):R1362–R1327. https://doi.org/10.1152/ajpregu.00470.2001
Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J (2001) Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A 98(17):9983–9988. https://doi.org/10.1073/pnas.171317998
Li L, Lai EY, Huang Y, Eisner C, Mizel D, Wilcox AC, Schnermann J (2012) Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors. Am J Physiol Ren Physiol 303(8):F1166–F1176. https://doi.org/10.1152/ajprenal.00222.2012
Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J (2004) Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest 114(5):634–642. https://doi.org/10.1172/JCI21851
Yao J, Suwa M, Li B, Kawamura K, Morioka T, Oite T (2003) ATP-dependent mechanism for coordination of intercellular Ca2+ signaling and renin secretion in rat juxtaglomerular cells. Circ Res 93(4):338–345. https://doi.org/10.1161/01.RES.0000086802.21850.5D
Vallon V, Mühlbauer B, Osswald H (2006) Adenosine and kidney function. Physiol Rev 86(3):901-40. https://doi.org/10.1152/physrev.00031.2005
Oppermann M, Friedman DJ, Faulhaber-Walter R, Mizel D, Castrop H, Enjyoji KE, Robson SC, Schnermann J (2008) Tubuloglomerular feedback and renin secretion in NTPDase1/CD39-deficient mice. Am J Physiol Ren Physiol 294(4):F965–F970. https://doi.org/10.1152/ajprenal.00603.2007
Koyner JL, Ceda J, Goldstein SL, Jaber BL, Liu KD, Shea JA, Faubel S, Acute Kidney Injury Advisory Group of the American Society of Nephrology (2014) The daily burden of acute kidney injury: a survey of U.S. nephrologists on world kidney day. Am J Kidney Dis 64:394–401. https://doi.org/10.1053/j.ajakd.2014.03.018
Malek M, Nematbacksh M (2015) Renal ischemia/reperfusion injury: from pathophysiology to treatment. J Renal Inj Prev 4(2):20–27. https://doi.org/10.12861/jrip.2015.06
Zuk A, Bonventre JV (2016) Acute kidney injury. Annu Rev Med 67(1):293–307. https://doi.org/10.1146/annurev-med-050214-013407
Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Müller CE, Robson SC, Osswald H, Eltzschig HK (2007) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J 21(11):2863–2873. https://doi.org/10.1096/fj.06-7947com
Crikis S, Ly B, Murray-Segal LM, Selan C, Robson SC, D’Apice AJ, Nandurkar HH, Cowan PJ, Dwyer KM (2010) Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant 10(12):2586–2595. https://doi.org/10.1111/j.1600-6143.2010.03257.x
Vincent IS, Okusa MD (2015) Adenosine 2A receptors in acute kidney injury. Acta Physiol (Oxf) 214(3):303–310. https://doi.org/10.1111/apha.12508
Rabadi MM, Lee HT (2015) Adenosine receptors and renal ischemia reperfusion injury. Acta Physiol (Oxf) 213(1):222–231. https://doi.org/10.1111/apha.12402
Sung SJ, Li L, Huang L, Lawler J, Ye H, Ronin DL, Vincent IS, Le TH, Yu J, Göridt N, Schrader J, Okusa MD (2016) Proximal tubule CD73 is critical in renal ischemia-reperfusion injury protection. J Am Soc Nephrol 28(3):888–902. https://doi.org/10.1681/ASN.2016020229
Cai M, Huttinger ZM, He H, Zhang W, Li F, Goodman LA, Wheeler DG, Druhan LJ, Zweler JL, Dwyer KM, He G, d’Apice AJF, Robson SC, Cowan PJ, Gumina RJ (2011) Transgenic over expression of ectonucleotide triphosphate diphosphohydroase-1 protects against murine myocardial ischemic injury. J Mol Cell Cardiol 51(6):927–935. https://doi.org/10.1016/j.yjmcc.2011.09.003
Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, Robson SC, d’Apice AJF, Cowan PJ, Dwyer KM (2013) Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology 57(4):1597–1606. https://doi.org/10.1002/hep.25985
Eltizschig HK, Köhler D, Eckle T, Kong T, Robson SC, Colgan SP (2009) Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113(1):224–232. https://doi.org/10.1182/blood-2008-06-165746
Sun X, Imai M, Nowak-Machen M, Guckelberger O, Enjyoji K, Wu Y, Khalpey Z, Berberat P, Munasinghe J, Robson SC (2011) Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia. Purinergic Signal 7(4):427–434. https://doi.org/10.1007/s11302-011-9239-6
Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltschig HK (2010) SP1-dependent induction of CD39 facilitates hepatic ischemic preconditions. J Immunol 184(7):4017–4024. https://doi.org/10.4049/jimmunol.0901851
Guckelberger O, Sun XF, Sévigny J, Imai M, Kaczmarek E, Enjyoji K, Kruskal JB, Robson SC (2004) Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost 91:576–586. https://doi.org/10.1160/TH03-06-0373
Jian R, Sun Y, Wang Y, Yu J, Zhong L, Zhou P (2011) CD73 protects kidney from ischemia-reperfusion injury through reduction of free radicals. Acta Pathol Microbiol Immunol Scand 120(2):130–138. https://doi.org/10.1111/j.1600-0463.2011.02827.x
Rajakumar SV, Lu B, Crikis S, Robson SC, d’Apice AJ, Cowan PJ, Dwyer KM (2010) Deficiency or inhibition of CD73 protects in mild kidney ischemia-reperfusion injury. Transplantation 90(12):1260–1264. https://doi.org/10.1097/TP.0b013e3182003d9b
Kim M, Ham A, Kim JY, Brown KM, D’Agati V, Lee HT (2013) The volatile anesthetic isoflurane induces ecto-5′-nucleotidases (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int 84(6):90–103. https://doi.org/10.1371/journal.pone.0099950
Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP (2006) HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J 20(13):2242–2250. https://doi.org/10.1096/fj.06-6419com
Roberts V, Lu B, Dwyer KM, Cowan PJ (2014) Adenosine receptor expression in the development of renal fibrosis following ischemic injury. Transplant Proc 46(10):3257–3261. https://doi.org/10.1016/j.transproceed.2014.09.151
Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK (2008) The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5(6):e137. https://doi.org/10.1371/journal.pmed.0050137
Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, ST J, Okusa MD (2009) Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20(8):1744–1753. https://doi.org/10.1681/ASN.2008111160
Zimmerman MA, Kam I, Eltzschig H, Grenz A (2013) Biological implications of extracellular adenosine in hepatic ischemia and reperfusion injury. Am J Transplant 13(10):2524–2529. https://doi.org/10.1111/ajt.12398
Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD (2010) Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int 77(9):771–780. https://doi.org/10.1038/ki.2010.12
Kinsey GR, Okusa MD (2013) Expanding role of T cells in acute kidney injury. Curr Opin Nephrol Hypertens 23(1):9–16. https://doi.org/10.1097/01.mnh.0000436695.29173.de
Kinsey GR, Huang L, Jaworska K, Khutsishivili K, Becker DA, Ye H, Lobo PI, Okusa MD (2012) Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephol 23(9):1528–1537. https://doi.org/10.1681/ASN.2012010070
Kinsey GR, Sharma R, Okusa MD (2013) Regulatory T cells in AKI. J Am Soc Nephrol 24(11):1720–1726. https://doi.org/10.1681/ASN.2013050502
Kinsey GR (2014) Macrophage dynamics in AKI to CKD progression. J Am Soc Nephrol 25:207–215. https://doi.org/10.1681/ASN2013101110
Liu YC, Zou XB, Chai YF, Yao YM (2014) Macrophage polarization in inflammatory diseases. Int J Biol Sci 10(5):520–529. https://doi.org/10.7150/ijbs.8879
Cao Q, Harris DC, Wang Y (2015) Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 30(3):183–194. https://doi.org/10.1152/physiol.00046.2014
Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA, Anders HJ (2014) Macrophage phenotype controls long-term AKI outcomes – kidney regeneration versus atrophy. J Am Soc Nephrol 25(2):292–304. https://doi.org/10.1681/ASN/2013020152
Duffiled JS (2014) Cellular and molecular mechanisms of kidney fibrosis. J Clin Invest 124(6):2299–2306. https://doi.org/10.1172/JCI72267
Nogueira A, Pires MJ, Oliveira PA (2017) Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In: In vivo, vol 31, pp 1–22. https://doi.org/10.21873/inivo.11019
Roberts VS, Cowan PJ, Alexander SI, Robson SC, Dwyer KM (2014) The role of adenosine receptors A2A and A2B signaling in renal fibrosis. Kidney Int 86(4):685–692. https://doi.org/10.1038/ki.2014.244
Roberts V, Campbell DJ, Lu B, Chia J, Cowan PJ, Dwyer KM (2017) The differential effect of apyrase treatment and hCD39 overexpression on chronic renal fibrosis following ischemia reperfusion injury. Transplantation 101(2017 Feb 14):e194–e204. https://doi.org/10.1097/TP.0000000000001679
Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y (2011) A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol 22(5):890–901. https://doi.org/10.1681/ASN.2010080890
Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Zhang W, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y (2013) Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112(11):1466–1478. https://doi.org/10.1161/CIRCRESAHA.111.300166
Ozüyaman B, Ding Z, Buchheiser A, Koszaika P, Braun N, Godecke A, Decking UK, Zimmermann H, Schrader J (2006) Adenosine produced via the CD73/ecto-5′-nucleotidase pathway has no impact on erythropoietin production but is associated with reduced kidney weight. Pflugers Arch 452(3):324–331. https://doi.org/10.1007/s00424-006-0045-x
Roberts V, Lu B, Chia J, Cowan PJ, Dwyer KM (2016) CD39 overexpression does not attenuate renal fibrosis in the unilateral ureteric obstructive model of chronic kidney disease. Purinergic Signal 12(4):653–660. https://doi.org/10.1007/s11302-016-9528-1
Lea-Henry T, Chacko B (2017) Management considerations in the failing renal allograft. Nephrology (Carlton). https://doi.org/10.1111/nep.13165
Malvezzi P, Rostaing L (2017) Renal transplantation in 2016. Novel approaches to improve recipient and allograft outcomes. Nat Rev Nephrol 13(2):73–74. https://doi.org/10.1038/nrneph.2016.190
Koziak K, Sévigny J, Robson SC, Siegel JB, Kaczmarek E (1999) Analysis of CD39/ATP disphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb Haemost 82(5):1538–1544
Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K (2005) Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost 31(02):217–233. https://doi.org/10.1055/s-2005-869527
Atkins B, Dwyer K, Enjyoji K, Robson SC (2006) Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential therapeutic targets. Blood Cells Mol Dis 36(2):217–222. https://doi.org/10.1016/j.bcmd.2005.12.025
Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC (2008) The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front Biosci 13:2588–2603
Salcido-Ochoa F, Tsang J, Tam P, Falk K, Rotzschke O (2010) Regulatory T cells in transplantation: does extracellular adenosine triphosphate metabolism through CD39 play a crucial role? Transplant Rev 24(2):52–66. https://doi.org/10.1016/j.trre.2010.01.002
Scalea J, Hanecamp I, Robson SC, Yamada K (2010) T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation 19:23–30. https://doi.org/10.1111/j.1399-3089.2011.00687.x.
Chernogorova P, Zeiser R (2012) Ectonucleotidases in solid organ and allogenic hematopoietic cell transplantation. J Biomed Biotechnol 2012:208204. https://doi.org/10.1155/208204
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 11(6):1257–1265. https://doi.org/10.1084/jem.20062512
Borsellino G, Kleinewietfield M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centronze D, Bernard D, Dell’Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk S (2007) Expression of ectonucleotidase CD39 by Fox3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110(4):1225–1232. https://doi.org/10.1182/blood-2006-12-064527
Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JI, Wingerhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB (2010) Expression of CD39 by human peripheral blood CD+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant 10(4):2410–2420. https://doi.org/10.1182/blood-2006-12-064527
McRae JL, Chia JS, Pommey SA, Dwyer KM (2016, 2016) Evaluation of CD4+ T cell populations in peripheral blood of patients following kidney transplantation and during acute allograft rejection. Nephrology. https://doi.org/10.1111/nep12894
Tang Q, Bleustone JA (2013) Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb Perpspect Med 3(11). https://doi.org/10.1101/cshperpect.1015552
Lunkes GI, Lunkes D, Stefanello F, Morsch A, Morsch VM, Mazzanti CM, Schetinger MR (2003) Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res 109:189–194. https://doi.org/10.1016/S0049-3848(03)00178-6
Lunkes GI, Lunkes DS, Leal D, Araujo Mdo C, Correa M, Becker L, Rosa CS, Morsch VM, Schetinger MR (2008) Effect of high glucose levels in human platelet NTPDase and 5′-nucleotidase activities. Diabetes Res Clin Pract 81(3):351–357. https://doi.org/10.1016/j.diabres.2008.06.001
Friedman DJ, Talbert ME, Bowden DW, Freedman BI, Mukanya Y, Enjyoji K, Robson SC (2009) Functional ENTPD1 polymorphisms in African Americans with diabetes and end-stage renal disease. Diabetes 58(4):999–1006. https://doi.org/10.2337/db08-1214
Karczewska J, Piwkowska A, Rogacka D, Stepinski J, Angielski S, Jankowsko M (2011) Purinergic modulation of glucose uptake into cultured rat podocytes: effect of diabetic milieu. Biochem Biophys Res Commun 404(2):723–727. https://doi.org/10.1016/j.bbrc.2010.12.051
Roa H, Gaiardo C, Troncoso E, Fuentealba V, Escudero C, Yañez A, Sobrevia L, Pastor-Anglada M, Quezada C, San Martin R (2009) Adenosine mediates transforming growth factor-beta 1 release in kidney glomeruli of diabetic rats. FEBS Lett 583(19):3192–3198. https://doi.org/10.1016/j.febslet.2009.09.003
Makino H, Miyamoto Y, Sawai K, Mori K, Mukoyama M, Nakao K, Yoshimasa Y, Suga S (2006) Altered gene expression related to glomerulogenesis and podocyte structure in early diabetic nephropathy of db/db mice and its restoration by pioglitazone. Diabetes 55(10):2747–2756. https://doi.org/10.2337/db05-1683
Roberts V, Stagg J, Dwyer KM (2014) The role of ectonucleotidases CD39 and CD73 and adenosine signaling in solid organ transplantation. Front Immunol 5. https://doi.org/10.3389/fimmun.2014.00064
Moeckel D, Jeong SS, Sun X, Broekman M, Nguyen A, Dorospouls JHF, Marcus AJ, Robson SC, Chen R, Abendschein D (2014) Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci Tranl Med 6:248ra105. https://doi.org/10.1126/scitranslamed.3009246
Al-Rashida M, Qazi SU, Batool N, Hameed A, Iqbal J (2017) Ectonucleotidase inhibitors: a patent review (2011-2016). Expert Opin Ther Pat:1–14. https://doi.org/10.1080/13543776.1369958
Geoghegan JC, Diedrich G, Lu X, Rosenthal K, Sachsenmeier KF, Wu H, Dall’Acqua WF, Damschroder MM (2016) Inhibition of CD73 AMP hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. mAbs 8:454–467. https://doi.org/10.1080/19420862.2016.1143182
Jacobson KA, Goa ZG (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Dis 5(3):247–264. https://doi.org/10.1038/nrd1983
Sachdeva S, Gupta M (2013) Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharma J 21:245–253. https://doi.org/10.1016/j.jsps.2012.05.011
Acknowledgements
Authors’ work cited in this review has been supported by grants from the US Department of Veterans Affairs (I01BX000596); US Department of Defense Peer Reviewed Medical Research Program (W81XWH-16-1-0464); the National Institutes of Health (R21 DK-081041, P01-HL107152, R21 CA-164970); the National Kidney Foundation of Utah and Idaho, and the resources and facilities at the Veterans Affairs Salt Lake City Health Care System (Salt Lake City, UT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclosures
BKK received research funding from AstraZeneca, AB; SCR has research funding from Tizona and Boston Biomedical.
Conflict of interest
Bellamkonda K. Kishore declares that he has no conflict of interest.
Simon C. Robson declares that he has no conflict of interest.
Karen M. Dwyer declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kishore, B.K., Robson, S.C. & Dwyer, K.M. CD39-adenosinergic axis in renal pathophysiology and therapeutics. Purinergic Signalling 14, 109–120 (2018). https://doi.org/10.1007/s11302-017-9596-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-017-9596-x