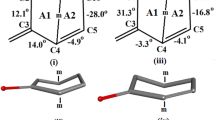
Abstract
Human aromatase is responsible for the last step of estrogen biosynthesis, for the aromatization of ring A of androstenedione or testosterone. In this work, the mechanism of aromatization was studied using gas phase and hybrid QM/MM calculations. It is shown that human aromatase can efficiently catalyze the aromatization process via a compound I (or compound II)-mediated pathway. The nature of the oxidant is very sensitive to the polarizing environment of the enzyme, as the oxidant has a compound I nature in the gas phase calculations, which is modulated by the enzyme environment to become a mixed compound I and compound II character. The electronic structure of the obtained QM-only and QM/MM stationary points is thoroughly discussed.















Similar content being viewed by others
References
Groves JT (2005) Models and mechanisms of cytochrome P450 action. In: Ortiz de Montellano PR (ed) Cytochrome P450, 3rd edn. Kluwer Academic/Plenum Publishers, New York, pp 1–43
Sligar SG (2010) Glimpsing the critical intermediate in cytochrome P450 oxidations. Science 330:924–925
Rittle J, Green MT (2010) Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science 330:933–937
Brodie AMH, Njar VCO (2000) Aromatase inhibitors and their application in breast cancer treatment. Steroids 65:171–179
Baum M, Budzar AU, Cuzik J, Forbes J, Houghton JH, Klijn JG, Sahmoud T (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC Randomised Trial. Lancet 359:2131–2139
Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M, Sahmoud T, Webster A, Steinberg M (2001) Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 92:2247–2258
Brueggemeier RW (2002) Aromatase inhibitors in breast cancer therapy. Expert Rev Anticancer Ther 2:181–191
Ryan KJ (1958) Conversion of androstenedione to estrone by placental microsomes. Biochim Biophys Acta 27:658–659
Ortiz de Montellano PR, Devoss JJ (2005) Substrate oxidation by cytochrome P450 enzymes. In: Ortiz de Montellano PR (ed) Cytochrome P450, 3rd edn. Kluwer Academic/Plenum Publishers, New York, pp 183–245
Groves JT, McClusky GA (1976) Aliphatic hydroxylation via oxygen rebound. oxygen transfer catalyzed by iron. J Am Chem Soc 98:859–861
Osawa Y, Shibata K, Rohrer D, Weeks C, Duax WL (1975) Reassignment of the Absolute Configuration of 19-Substituted 19-Hydroxysteroids and Stereomechanism of Estrogen Biosynthesis. J Am Chem Soc 97:4400–4402
Arigoni D, Battaglia R, Akhtar M, Smith T (1975) Stereospecificity of oxidation at C-19 in oestrogen biosynthesis. J Chem Soc, Chem Commun 6:185–186
Hosoda H, Fishman J (1974) Unusually facile aromatization of 2.beta.-hydroxy-19-oxo-4-androstene-3,17-dione to estrone: implications in estrogen biosynthesis. J Am Chem Soc 96:7325–7329
Goto J, Fishman J (1977) Participation of a nonenzymatic transformation in the biosynthesis of estrogens from androgens. Science 195:80–81
Hahn EF, Fishman J (1984) Immunological probe of estrogen biosynthesis: evidence for the 2 beta-hydroxylative pathway in aromatization of androgens. J Biol Chem 259:1689–1694
Morand P, Williamson DG, Layne DS, Lompa-Krzymien L, Salvador J (1975) Conversion of an androgen epoxide into 17β-estradiol by human placental microsomes. Biochemistry 14:635–638
Mastalerz H, Morand P (1982) Acid- and base-catalysed reactions of 4β,5β- and 4α,5α-epoxyandrostane-3,17,19-trione. J Chem Soc, Perkin Trans 1:2611–2615
Brodie HJ, Kripalani KJ, Possanza G (1969) Mechanism of estrogen biosynthesis. VI. The stereochemistry of hydrogen elimination at C-2 during aromatization. J Am Chem Soc 91:1241–1242
Fishman J, Guzik H, Dixon D (1969) Stereochemistry of estrogen biosynthesis. Biochemistry 8:4304–4309
Akhtar M, Calder MR, Corina DL, Wright JN (1982) Mechanistic studies on C-19 demethylation in oestrogen biosynthesis. Biochem J 201:569–580
Gantt SL, Denisov IG, Grinkova YV, Sligar SG (2009) The critical iron-oxygen intermediate in human aromatase. Biochem Biophys Res Commun 387:169–173
Davydov R, Makris TM, Kofman V, Werst DE, Sligar SG, Hoffman BM (2001) Hydroxylation of camphor by reduced oxy-cytochrome P450cam: mechanistic implications of EPR and ENDOR studies of catalytic intermediates in native and mutant enzymes. J Am Chem Soc 123:1403–1415
Graham-Lorence S, Amarneh B, White RE, Peterson JA, Simpson ER (1995) A three-dimensional model of aromatase cytochrome P450. Protein Sci 4:1065–1080
Tosha T, Kagawa N, Ohta T, Yoshioka S, Waterman MR, Kitagawa T (2006) Raman evidence for specific substrate-induced structural changes in the heme pocket of human cytochrome P450 aromatase during the three consecutive oxygen activation steps. Biochemistry 45:5631–5640
Hackett JC, Brueggemeier RW, Hadad CM (2005) The final catalytic step of cytochrome P450 aromatase: a density functional theory study. J Am Chem Soc 127:5224–5237
Sen K, Hackett JC (2012) Coupled electron transfer and proton hopping in the final step of CYP19-catalyzed androgen aromatization. Biochemistry 51:3039–3049
Mak PJ, Luthra A, Sligar SG, Kincaid JR (2014) Resonance Raman spectroscopy of the oxygenated intermediates of human CYP19A1 implicates a Compound I intermediate in the final lyase step. J Am Chem Soc 136:4825–4828
Khatri Y, Luthra A, Duggal R, Sligar SG (2014) Kinetic solvent isotope effect in steady-state turnover by CYP19A1 suggests involvement of Compound 1 for both hydroxylation and aromatization steps. FEBS Lett 588:3117–3122
Shaik S, Kumar D, de Visser SP, Altun A, Thiel W (2005) Theoretical Perspective on the Structure and Mechanism of Cytochrome P450 Enzymes. Chem Rev 105:2279–2328
Shaik S, Cohen S, Wang Y, Chen H, Kumar D, Thiel W (2010) P450 enzymes: their structure, reactivity, and selectivity—modeled by QM/MM calculations. Chem Rev 110:949–1017
Sivaramakrishnan S, Ouellet H, Matsumura H, Guan S, Moënne-Loccoz P, Burlingame AL, Ortiz de Montellano PR (2012) Proximal ligand electron donation and reactivity of the cytochrome P450 ferric-peroxo anion. J Am Chem Soc 134:6673–6684
Ouellet H, Guan S, Johnston JB, Chow ED, Kells PM, Burlingame AL, Cox JS, Podust LM, de Montellano PR (2010) Mycobacterium tuberculosis CYP125A1, a steroid C27 monooxygenase that detoxifies intracellularly generated cholest-4-en-3-one. Mol Microbiol 77:730–742
van der Kamp MW, Mulholland AJ (2013) Combined quantum mechanics/molecular mechanics (QM/MM) methods in computational enzymology. Biochemistry 52:2708–2728
Singh UC, Kollman PA (1986) A combined Ab Iinitio quantum mechanical and molecular mechanical method for carrying out simulations on complex molecular systems: applications to the CH3Cl + Cl− exchange reaction and gas phase protonation of polyethers. J Comput Chem 7:718–730
Warshel A, Levitt M (1976) Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol 103:227–249
Field MJ, Bash PA, Karplus M (1990) A combined quantum mechanical and molecular mechanical potential for molecular dynamics simulations. J Comput Chem 11:700–733
Thèry V, Rinaldi D, Rivail J-L, Maigret B, Ferenczy GG (1994) Quantum mechanical computations on very large molecular systems: The local self-consistent field method. J Comput Chem 15:269–282
Ferenczy Gy G (2013) Calculation of wave-functions with frozen orbitals in mixed quantum mechanics/molecular mechanics methods. Part I. Application of the Huzinaga equation. J Comput Chem 34:854–861
Ferenczy Gy G (2013) Calculation of wave-functions with frozen orbitals in mixed quantum mechanics/molecular mechanics methods. Part II. Application of the local basis equation. J Comput Chem 34:862–869
Kästner J, Thiel S, Senn HM, Sherwood P, Thiel W (2007) Exploiting QM/MM capabilities in geometry optimization: a microiterative approach using electrostatic embedding. J Chem Theory Comput 3:1064–1072
Kumar S, Rosenberg JM, Bouzida D, Swendsen RH, Kollman PA (1992) The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J Comput Chem 13:1011–1021
Prasad BR, Plotnikov NV, Lameira J, Warshel A (2013) Quantitative exploration of the molecular origin of the activation of GTPase. Proc Natl Acad Sci USA 110:20509–20514
Plotnikov NV, Prasad BR, Chakrabarty S, Chu ZT, Warshel A (2013) Quantifying the mechanism of phosphate monoester hydrolysis in aqueous solution by evaluating the relevant Ab Initio QM/MM free-energy surfaces. J Phys Chem B 117:12807–12819
Laio A, Parrinello M (2002) Escaping free-energy minima. Proc Natl Acad Sci USA 99:12562–12566
Plotnikov NV, Kamerlin SC, Warshel A (2011) Paradynamics: an effective and reliable model for Ab Initio QM/MM free-energy calculations and related tasks. J Phys Chem B 115:7950–7962
Krámos B, Oláh J (2014) Enolization as an alternative proton delivery pathway in human aromatase (P450 19A1). J Phys Chem B 118:390–405
Zheng JJ, Wang D, Thiel W, Shaik S (2006) QM/MM study of mechanisms for compound I formation in the catalytic cycle of cytochrome P450cam. J Am Chem Soc 128:13204–13215
Groves JT, Gross Z, Stern MK (1994) Preparation and reactivity of oxoiron(IV) porphyrins. Inorg Chem 33:5065–5072
He K, Falick AM, Chen B, Nilsson F, Correia MA (1996) Identification of the heme adduct and an active site peptide modified during mechanism-based inactivation of rat liver cytochrome P450 2B1 by secobarbital. Chem Res Toxicol 9:614–622
de Visser SP, Kumar D, Shaik S (2004) How do aldehyde side products occur during alkene epoxidation by cytochrome P450? Theory reveals a state-specific multi-state scenario where the high-spin component leads to all side products. J Inorg Biochem 98:1183–1193
Ogliaro F, Cohen S, de Visser SP, Shaik S (2000) Medium polarization and hydrogen bonding effects on compound I of cytochrome P450: what kind of a radical is it really? J Am Chem Soc 122:12892–12893
Ogliaro F, de Visser SP, Cohen S, Kaneti J, Shaik S (2001) The experimentally elusive oxidant of cytochrome P450: a theoretical, “trapping” defining more closely the “real” species. ChemBioChem 2:848–851
Bathelt CM, Zurek J, Mulholland AJ, Harvey JN (2005) Electronic structure of compound i in human isoforms of cytochrome P450 from QM/MM modeling. J Am Chem Soc 127:12900–12908
Ogliaro F, Cohen S, Filatov M, Harris N, Shaik S (2000) The high-valent compound of cytochrome P450: the nature of the Fe–S bond and the role of the thiolate ligand as an internal electron donor. Angew Chem Int Ed 39:3851–3855
Ogliaro F, Cohen S, Filatov M, Harris N, Shaik S (2001) The high-valent compound of cytochrome P450: the nature of the Fe–S bond and the role of the thiolate ligand as an internal electron donor. Angew Chem Int Ed 40:647
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian 09, revision A.1. Gaussian Inc, Wallingford, CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Altun A, Kumar D, Neese F, Thiel W (2008) Multireference Ab Initio quantum mechanics/molecular mechanics study on intermediates in the catalytic cycle of cytochrome P450cam. J Phys Chem A 112:12904–12910
Dolg M, Wedig U, Stoll H, Preuss H (1987) Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys. 86:866–872
Andrae D, Haussermann U, Dolg M, Stoll H, Preuss H (1990) Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor Chim Acta 77:123–141
Krámos B, Menyhárd DK, Oláh J (2012) Direct hydride shift mechanism and stereoselectivity of P450nor confirmed by QM/MM calculations. J Phys Chem B 116:872–885
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
NBO 5.9, Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales C, Weinhold F (2009) Theoretical Chemistry Institute, University of Wisconsin, Madison, WI, 2009. http://www.chem.wisc.edu/~nbo5. Accessed Nov 4, 2013
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Tao JM, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. a direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem Phys 55:117–129
Pascual-Ahuir JL, Silla E, Tuñón I (1994) GEPOL: an improved description of molecular-surfaces. 3. A new algorithm for the computation of a solvent-excluding surface. J Comp Chem 15:1127–1138
Ghosh D, Griswold J, Erman M, Pangborn W (2009) Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457:219–223
Altun A, Guallar V, Friesner RA, Shaik S, Thiel W (2006) The effect of heme environment on the hydrogen abstraction reaction of camphor in P450cam catalysis: a QM/MM study. J Am Chem Soc 128:3924–3925
Altun A, Shaik S, Thiel W (2006) Systematic QM/MM investigation of factors that affect the cytochrome P450-catalyzed hydrogen abstraction of camphor. J Comput Chem 27:1324–1337
MacKerell AD Jr, Banavali N, Foloppe N (2000) Development and current status of the CHARMM Force field for nucleic acids. Biopolymers 56:257–265
Brooks BR, Brooks CL, Mackerell AD, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S et al (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30:1545–1614
Harvey JN (2004) Spin-forbidden CO ligand recombination in myoglobin. Faraday Discuss. 127:165–177
TINKER—Home Page. Tinker—software tools for molecular design. http://dasher.wustl.edu/tinker/. Accessed October 5, 2011
Ren P, Wu C, Ponder JW (2011) Polarizable atomic multipole-based molecular mechanics for organic molecules. J Chem Theory Comput 7:3143–3161
Lonsdale R, Harvey JN, Mulholland AJ (2010) Inclusion of dispersion effects significantly improves accuracy of calculated reaction barriers for cytochrome P450 catalyzed reactions. J Phys Chem Lett 1:3232–3237
Lonsdale R, Harvey JN, Mulholland AJ (2012) Effects of dispersion in density functional based quantum mechanical/molecular mechanical calculations on cytochrome P450 catalyzed reactions. J Chem Theory Comput 8:4637–4645
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Schaftenaar G, Noordik JH (2000) Molden: a pre- and post-processing program for molecular and electronic structures. J Comput-Aided Mol Design 14:123–134
Humphrey W, Dalke A, Schulten K (1996) VMD - Visual Molecular Dynamics. J Mol Graphics 14:33–38
Schöneboom JC, Cohen S, Lin H, Shaik S, Thiel W (2004) Quantum mechanical/molecular mechanical investigation of the mechanism of C–H hydroxylation of camphor by cytochrome P450cam: theory supports a two-state rebound mechanism. J Am Chem Soc 126:4017–4034
Meigs RA, Ryan KJ (1971) Enzymatic aromatization of steroids I. Effects of oxygen and carbon monoxide on the intermediate steps of estrogen biosynthesis. J Biol Chem 246:83–87
Townsley JD, Brodi HJ (1968) Mechanism of estrogenbiosynthesis. III. Stereochemistry of aromatization of C19 and C18 steroids. Biochemistry 7:33–40
Conradie J, Abhik Ghosh A (2007) DFT calculations on the spin-crossover complex Fe(salen)(NO): a quest for the best functional. J Phys Chem B 111:12621–12624
Harvey JN (2006) On the accuracy of density functional theory in transition metal chemistry. Annu Rep Prog Chem C 102:203–226
Radoń M (2014) Revisiting the role of exact exchange in DFT spin-state energetics of transition metal complexes. Phys Chem Chem Phys 16:14479–14488
Frushicheva MP, Warshel A (2012) Towards quantitative computer-aided studies of enzymatic enantioselectivity: the case of candida Antarctica lipase A. ChemBioChem 13:215–223
Lonsdale R, Hoyle S, Grey DT, Ridder L, Mulholland AJ (2012) Determinants of reactivity and selectivity in soluble epoxide hydrolase from quantum mechanics/molecular mechanics modeling. Biochemistry 51:1774–1786
Torrie GM, Valleau JP (1974) Monte Carlo free energy estimates using non-Boltzmann sampling: application to the sub-critical Lennard-Jones fluid. Chem Phys Lett 28:578–581
Car R, Parrinello M (1985) Unified approach for molecular dynamics and density-functional theory. Phys Rev Lett 55:2471–2474
Ufimtsev IS, Martínez TJ (2008) Quantum chemistry on graphical processing units. 1. Strategies for two-electron integral evaluation. J Chem Theory Comput 4:222–231
Sisto A, Glowacki DR, Martinez TJ (2014) Ab Initio nonadiabatic dynamics of multichromophore complexes: a scalable graphical-processing-unit-accelerated exciton framework. Acc Chem Res 47:2857–2866
Lonsdale R, Houghton KT, Żurek J, Bathelt CM, Foloppe N, de Groot MJ, Harvey JN, Mulholland AJ (2013) Quantum mechanics/molecular mechanics modeling of regioselectivity of drug metabolism in cytochrome P450 2C9. J Am Chem Soc 135:8001–8015
Oláh J, Mulholland AJ, Harvey JN (2011) Understanding the Determinants of Selectivity in Drug Metabolism through Modeling of Dextromethorphan Oxidation by Cytochrome P450. Proc Natl Acad Sci USA 108:6050–6055
Ranaghan KE, Mulholland AJ (2010) Investigations of enzyme-catalysed reactions with combined quantum mechanics/molecular mechanics (QM/MM) methods. Int Rev Phys Chem 29:65–133
Klähn M, Braun-Sand S, Rosta E, Warshel A (2005) On possible pitfalls in ab initio quantum mechanics/molecular mechanics minimization approaches for studies of enzymatic reactions. J Phys Chem B 109:15645–15650
Harvey JN, Bathelt CM, Mulholland AJ (2006) QM/MM modeling of compound I active species in cytochrome P450, cytochrome C peroxidase, and ascorbate peroxidase. J Comput Chem 27:1352–1362
Warshel A, Sharma PK, Kato M, Xiang Y, Liu H, Olsson MH (2006) Electrostatic basis for enzyme catalysis. Chem Rev 106:3210–3235
Warshel A (1978) Energetics of enzyme catalysis. Proc Natl Acad Sci USA 75:5250–5254
Acknowledgments
The authors thank Prof. Jeremy Harvey (University of Bristol. UK) for useful discussions, Dóra K. Menyhárd for proof-reading of the manuscript, and the financial support of the New Széchenyi Plan (TÁMOP-4.2.2/B-10/1-2010-0009) and of OTKA Grant No. 108721. JO acknowledges receipt of an EU Marie Curie ERG Fellowship (Project “Oestrometab”). Part of this work was supported by the COST Action CM1305 (ECOSTBio).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11224_2014_545_MOESM1_ESM.doc
SUPPORTING INFORMATION. Selected geometrical parameters and fragment charges for QM-only optimized structures using various basis sets, and details of all reactants, TS, and product complexes obtained in QM/MM calculations. Furthermore, total energies and Cartesian coordinates of all QM-only structures, geometries of the QM region of the QM/MM structures belonging to profile 1, and MM parameters for the 19-oxo-ASD are also provided in the SI together with tables and figures indicated in the text. (DOC 2789 kb)
Rights and permissions
About this article
Cite this article
Krámos, B., Oláh, J. The mechanism of human aromatase (CYP 19A1) revisited: DFT and QM/MM calculations support a compound I-mediated pathway for the aromatization process. Struct Chem 26, 279–300 (2015). https://doi.org/10.1007/s11224-014-0545-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0545-9