Abstract
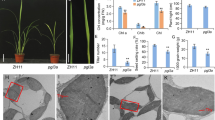
The consequences of ketocarotenoid production in transgenic tobacco (Nicotiana tabacum) plants expressing a Chlamydomonas reinhardtii gene encoding a β-carotene ketolase were examined concerning the functionality of the photosynthetic apparatus. T1 plants produced less photosynthetic pigments per dry weight, but Chl a/Chl b ratios remained unchanged. Almost as much ketocarotenoids as accessory xanthophylls accumulated per Chl a molecule. These ketocarotenoids were found mainly in the thylakoid membranes, but were not functionally bound to light-harvesting complexes, although LHCII is known to be able to bind astaxanthin. On the contrary, high amounts of ketocarotenoids probably changed the properties of the lipid phase of the thylakoids, thereby reducing the stability of photosystem II supercomplexes and LHCII trimers and ultimately decreasing grana formation. In addition, photosystem II function in electron transport was impaired, and plants exhibited less non-photochemical quenching compared to wild-type plants. Thus, in order not to disturb vital functions of the plants, production of astaxanthin and other nutritionally valuable ketocarotenoids apparently requires ways to sequester the additional carotenoids to plastoglobuli.





Similar content being viewed by others
Abbreviations
- Bkt:
-
β-carotene ketolase
- BBY:
-
Grana membranes
- Chl:
-
Chlorophyll
- LHCII:
-
Light-harvesting complex II
- PS:
-
Photosystem
- NPQ:
-
Non-photochemical quenching
- RbcS:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase, small subunit
- WT:
-
Wild type
References
Barber J (1986) Regulation of energy transfer by cations and protein phosphorylation in relation to thylakoid membrane organization. Photosynth Res 10:243–253
Beer A, Gundermann K, Beckmann J et al (2006) Subunit composition and pigmentation of fucoxanthin-chlorophyll proteins in diatoms: evidence for a subunit involved in diadinoxanthin and diatoxanthin binding. Biochemistry 45:13046–13053
Berthold DA, Babcock GT, Yocum CF (1981) A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett 134:231–234
Betterle N, Ballottari M, Zorzan S et al (2009) Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J Biol Chem 284:15255–15266
Breitenbach J, Misawa N, Kajiwara S et al (1996) Expression in Escherichia coli and properties of the carotene ketolase from Haematococcus pluvialis. FEMS Microbiol Lett 140:241–246
Caffarri S, Kouřil R, Kereïche S et al (2009) Functional architecture of higher plant photosystem II supercomplexes. EMBO J 28:3052–3063
Collins AM, Jones HDT, Han D et al (2011) Carotenoid distribution in living cells of Haematococcus pluvialis (Chlorophyceae). PLoS One 6:e24302
Croce R, Weiss S, Bassi R (1999) Carotenoid-binding sites of the major light-harvesting complex of higher plants. J Biol Chem 274:29613–29623
Dietzel L, Bräutigam K, Steiner S et al (2011) Photosystem II supercomplex remodeling serves as an entry mechanism for state transitions in Arabidopsis. Plant Cell 23:2964–2977
Formaggio E, Cinque G, Bassi R (2001) Functional architecture of the major light-harvesting complex from higher plants. J Mol Biol 314:1157–1166
Genty B, Briantais JM, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Han D, Li Y, Hu Q (2013) Astaxanthin in microalgae: pathways, functions and biotechnological implications. Algae 28:131–147
Hasunuma T, Miyazawa S, Yoshimura S et al (2008) Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J 55:857–868
Holt NE, Zigmantas D, Valkunas L et al (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307:433–436
Huang J, Zhong Y, Sandmann G et al (2012) Cloning and selection of carotenoid ketolase genes for the engineering of high-yield astaxanthin in plants. Planta 236:691–699
Jeffrey SW, Mantoura RFC, Bjørnland T (1997) Data for the identification of 47 key phytoplankton pigments. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO Pub, Paris
Kereïche S, Kiss AZ, Kouřil R et al (2010) The PsbS protein controls the macro-organisation of photosystem II complexes in the grana membranes of higher plant chloroplasts. FEBS Lett 584:759–764
Kim S, Schlicke H, van Ree K et al (2014) Arabidopsis chlorophyll biosynthesis: an essential balance between the methylerythritol phosphate and tetrapyrrole pathways. Plant Cell 25:4984–4993
Lazár D (2006) The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct Plant Biol 33:9–30
Liu Z, Yan H, Wang K et al (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428:287–292
Lokstein H, Tian L, Polle JEW et al (2002) Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in photosystem II antenna size and stability. Biochim Biophys Acta 1553:309–319
Mishra Y, Jänkänpää HJ, Kiss AZ et al (2012) Arabidopsis plants grown in the field and climate chambers significantly differ in leaf morphology and photosystem components. BMC Plant Biol 12:6
Mulders KJM, Lamers PP, Martens DE et al (2014) Phototrophic pigment production with microalgae: biological constraints and opportunities. J Phycol 50:229–242
Nussberger S, Dörr K, Wang DN et al (1993) Lipid-protein interactions in crystals of plant light-harvesting complex. J Mol Biol 234:347–356
Pascal AA, Liu Z, Broess K et al (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436:134–137
Phillip D, Hobe S (2002) The binding of xanthophylls to the bulk light-harvesting complex of photosystem II of higher plants. A specific requirement for carotenoids with a 3-hydroxy-beta-end group. J Biol Chem 277:25160–25169
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Ruban AV, Berera R, Ilioaia C et al (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450:575–579
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Schägger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199:223–231
Schansker G, Tóth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta 1706:250–261
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Standfuss J, van Scheltinga ACT, Lamborghini M et al (2005) Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J 24:919–928
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol B 104:236–257
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Trémoliéres A, Dubacq JP, Ambard-Bretteville F et al (1981) Lipid composition of chlorophyl–protein complexes: specific enrichment in trans-hexadecenoic acid of an oligomeric form of light-harvesting chlorophyll a/b protein. FEBS Lett 130:27–31
Vechtel B, Pistorius EK, Ruppel HG (1992) Occurence of secondary carotenoids in PS I complexes isolated from Eremosphaera viridis (De Bary) (Chlorophyceae). Z Naturforsch C 47:51–56
Wahadoszamen M, Berera R, Ara AM et al (2012) Identification of two emitting sites in the dissipative state of the major light harvesting antenna. Phys Chem Chem Phys 14:759–766
Wilk L, Grunwald M, Liao P et al (2013) Direct interaction of the major light-harvesting complex II and PsbS in nonphotochemical quenching. Proc Natl Acad Sci USA 110:5452–5456
Zheleva D, Sharma J, Panico M et al (1998) Isolation and characterization of monomeric and dimeric CP47-reaction center photosystem II complexes. J Biol Chem 273:16122–16127
Zhu C, Naqvi S, Capell T et al (2009) Metabolic engineering of ketocarotenoid biosynthesis in higher plants. Arch Biochem Biophys 483:182–190
Acknowledgments
We thank Prof. Feng Chen, Bejing University and Dr.Junchao Huang, Chinese Academy of Sciences, Kunming, China, for kindly providing the seeds of the transgenic tobacco plants. ChristophJedmowski and Prof. Wolfgang Brüggemann are acknowledged for providing O−J−I–P equipment and for help with the data analysis. The work is part of A.R.’s Ph. D thesis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Röding, A., Dietzel, L., Schlicke, H. et al. Production of ketocarotenoids in tobacco alters the photosynthetic efficiency by reducing photosystem II supercomplex and LHCII trimer stability. Photosynth Res 123, 157–165 (2015). https://doi.org/10.1007/s11120-014-0055-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0055-z