Purpose
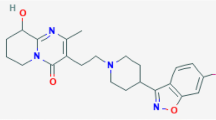
The bioavailability of a development candidate active pharmaceutical ingredient (API) was very low after oral dosing in dogs. In order to improve bioavailability, we sought to increase the dissolution rate of the solid form of the API. When traditional methods of forming salts and amorphous material failed to produce a viable solid form for continued development, we turned to the non-traditional approach of cocrystallization.
Methods
A crystal engineering approach was used to design and execute a cocrystal screen of the API. Hydrogen bonding between the API and pharmaceutically acceptable carboxylic acids was identified as a viable synthon for associating multiple components in the solid state. A number of carboxylic acid guest molecules were tested for cocrystal formation with the API.
Results
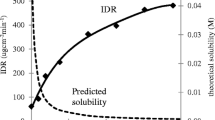
A cocrystal containing the API and glutaric acid in a 1:1 molecular ratio was identified and the single crystal structure is reported. Physical characterization of the cocrystal showed that it is unique regarding thermal, spectroscopic, X-ray, and dissolution properties. The cocrystal solid is nonhygroscopic, and chemically and physically stable to thermal stress. Use of the cocrystal increased the aqueous dissolution rate by 18 times as compared to the homomeric crystalline form of the drug. Single dose dog exposure studies confirmed that the cocrystal increased plasma AUC values by three times at two different dose levels.
Conclusions
APIs that are non-ionizable or demonstrate poor salt forming ability traditionally present few opportunities for creating crystalline solid forms with desired physical properties. Cocrystals are an additional class of crystalline solid that can provide options for improved properties. In this case, a crystalline molecular complex of glutaric acid and an API was identified and used to demonstrate an improvement in the oral bioavailability of the API in dogs.












Similar content being viewed by others
References
G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–419 (1995).
Calculated using Advanced Chemistry Development (ACD/Labs) Software Solaris V4.67 (1994–2005 ACD/Labs).
M. Yazdanian, S. L. Glynn, J. L. Wright, and A. Hawi. Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm. Res. 15(9):1490–1494 (1998).
S. L. Childs, L. C. Chyall, J. T. Dunlap, V. N. Smolenskaya, B. C. Stahly, and G. P. Stahly. Crystal engineering approach to forming cocrystals of amine hydrochlorides with organic acids. Molecular complexes of fluoexetine hydrochloride with benzoic, succinic, and fumaric acids. J. Am. Chem. Soc. 126:13335–13342 (2004).
J. F. Remenar, S. L. Morissette, M. L. Peterson, B. Moulton, J. M. MacPhee, H. R. Guzman, and O. Almarsson. Crystal engineering of novel cocrystals of a triazole drug with 1,4-dicarboxylic acids. J. Am. Chem. Soc. 125:8456–8457 (2003).
J. W. Bettis, J. L. Lach, and J. Hood. Effect of complexation with phenobarbital on biologic availability of theophylline from 3 tablet formulations. Am. J. Hosp. Pharm. 30(3):240–243 (1973).
J. Bernstein, M. C. Etter, and L. Leiserowitz. The role of hydrogen bonding in molecular assemblies. In H.-B. D. Buergi, and Jack D (eds.), Struct. Correl., VCH, Weinheim, Germany, 1994, pp. 431–507.
I. D. H. Oswald, D. R. Allan, P. A. McGregor, W. D. S. Motherwell, S. Parsons, and C. R. Pulham. The formation of paracetamol (acetaminophen) adducts with hydrogen-bond acceptors. Acta Crystallogr. Sect. B-Struct. Commun. 58:1057–1066 (2002).
N. Sardone, G. Bettinetti, and M. Sorrenti. Trimethoprim-sulfadimidine 1:2 molecular complex monohydrate. Acta Crystallogr., C Cryst. Struct. Commun. 53:1295–1299 (1997).
S. Nakao, S. Fujii, T. Sakaki, and K. I. Tomita. Crystal and molecular-structure of 2-1 molecular-complex of theophylline with phenobarbital. Acta Crystallogr. Sect. B-Struct. Commun. 33:1373–1378 (1977) (MAY13).
M. R. Caira, T. G. Dekker, and W. Liebenberg. Structure of a 1:1 complex between the anthelmintic drug mebendazole and propionic acid. J. Chem. Crystallogr. 28(1):11–15 (1998).
M. C. Etter, and G. M. Frankenbach. Hydrogen-bond directed cocrystallization as a tool for designing acentric organic solids. Chem. Mater. 1(1):10–12 (1989).
C. B. Aakeroy. Crystal engineering: strategies and architectures. Acta Crystallogr. Sect. B-Struct. Commun. 53:569–586 (1997).
M. C. Etter and D. A. Adsmond. The use of cocrystallization as a method of studying hydrogen-bond preferences of 2-aminopyrimidine. J. Chem. Soc., Chem. Commun. 8:589–591 (1990).
G. R. Desiraju. Supramolecular synthons in crystal engineering—a new organic-synthesis. Angew. Chem.-Int. Edit. Engl. 34(21):2311–2327 (1995).
A. Nangia and G. R. Desiraju. Supramolecular structures—reason and imagination. Acta Crystallogr. Sect. A 54:934–944 (1998).
B. Rodriguez-Spong, C. P. Price, A. Jayasankar, A. J. Matzger, and N. Rodriguez-Hornedo. General principles of pharmaceutical solid polymorphism: a supramolecular perspective. Adv. Drug Deliv. Rev. 56(3):241–274 (2004).
J. J. Kane, T. Nguyen, J. Xiao, F. W. Fowler, and J. W. Lauher. The host guest co-crystal approach to supramolecular structure. Mol. Cryst. Liquid Cryst. 356:449–458 (2001).
P. Vishweshwar, A. Nangia, and V. M. Lynch. Molecular complexes of homologous alkanedicarboxylic acids with isonicotinamide: X-ray crystal structures, hydrogen bond synthons, and melting point alternation. Cryst. Growth Des. 3(5):783–790 (2003).
P. Vishweshwar, A. Nangia, and V. M. Lynch. Supramolecular synthons in phenol-isonicotinamide adducts. Crystengcomm.: 164–168 (2003).
M. C. Etter. Aggregate structures of carboxylic acids and amides. Isr. J. Chem. 25(3–4):312–319 (1985).
SMART Version 5.55, 2000, Bruker AXS, Inc., Analytical X-ray Systems, 5465 East Cheryl Parkway, Madison Wisconsin 53711–5373.
SAINT Version 6.02, 1999, Bruker AXS, Inc., Analytical X-ray Systems, 5465 East Cheryl Parkway, Madison Wisconsin 53711–5373.
SHELXTL V5.10, 1997, Bruker AXS, Inc., Analytical X-ray Systems, 5465 East Cheryl Parkway, Madison Wisconsin 53711–5373.
A. J. C. Wilson (ed.), International Tables for X-ray Crystallography, Volume C. Kynoch, Academic, Dordrecht, 1992, Tables 6.1.1.4 (pp. 500–502) and 4.2.6.8 (pp. 219–222).
C. G. S. Wermuth. P. H. Handbook of Pharmaceutical Salts; Properties, Selection, and Use (P. H. W. Stahl, C. G., ed.). Verlag Helvitica Chimica Acta, Zurich and Wiley-VCH: Weinheim, 306 (2002).
W. C. McCrone, Jr., Fusion Methods in Chemical Microscopy. Interscience, New York, 1957.
A. Kofler. Behavior of crystalline solid solution during melting and crystallization. Mikroskopie. 11(5–6):140–155 (1956).
M. Kunhert-Brandstaetter. 40 Years of Kofler methods. Pharma Int. (Engl. Ed.) 5:5–11 (1971).
R. N. Rai, and K. B. R. Varma. Phase diagram and dielectric studies of binary organic materials. Mater. Lett. 44(5):284–293 (2000).
U. S. Rai and S. George. Some thermochemical studies on binary faceted organic eutectics and 1:1 molecular complexes. J. Therm. Anal. 46(6):1809–1820 (1996).
N. R. Jagannathan and C. N. R. Rao. A 13C NMR spectroscopic study of the phase transitions of alkane dicarboxylic acids in the solid state. Chem. Phys. Lett. 140(1):46–50 (1987).
S. J. Nehm, B. Rodriguez-Spong, and N. Rodriguez-Hornedo. Phase solubility diagrams of cocrystals are explained by solubility product and solution complexation. Cryst. Growth Des. 6(2):592–600 (2006).
Acknowledgments
The authors thank Dr. Ken Hardcastle at the Emory University Chemistry Department X-Ray Diffraction Center for collecting and solving the single crystal structure of the cocrystal. The authors also acknowledge the support of Drs. Phil Goliber and Leah Lipsich of Purdue Pharma L. P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McNamara, D.P., Childs, S.L., Giordano, J. et al. Use of a Glutaric Acid Cocrystal to Improve Oral Bioavailability of a Low Solubility API. Pharm Res 23, 1888–1897 (2006). https://doi.org/10.1007/s11095-006-9032-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9032-3