Abstract
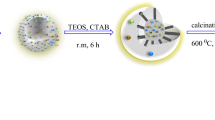
Monodispersed and uniform Au@mesoporous SnO2 yolk–shell nanoparticles (Au@mSnO2 yolk–shell NPs) composed of the moveable Au NP cores and mSnO2 shells have been successfully fabricated via a facile and reproducible approach. The outside mSnO2 shells of Au@mSnO2 yolk–shell NPs not only prevent Au NPs from aggregating and corroding by the reaction solution but also allow the Au NPs to contact with reactant molecules easily through the mesoporous channels. The obtained Au@mSnO2 yolk–shell NPs are characterized by means of transmission electron microscope, scanning electron microscopy, X-ray powder diffraction, X-ray photoelectron spectrum, and UV–vis absorption spectroscopy. The synthesized materials exhibit excellent catalytic performance and high stability towards the reduction of 4-nitrophenol with NaBH4 as a reducing agent, which may be ascribed to their high specific surface area and unique mesoporous structure. Moreover, the synthetic strategy reported in this paper can be extended to fabricate a series of multifunctional noble metal@metal oxide yolk–shell nanocomposite materials with unique properties for various applications.








Similar content being viewed by others
References
Ansari SA, Khan MM, Ansari MO, Lee J, Cho MH (2014) Highly photoactive SnO2 nanostructures engineered by electrochemically active biofilm. New J Chem 38:2462–2469
Chang YC, Chen DH (2009) Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst. J Hazard Mater 165:664–669
Chen JC, Zhang RY, Han L, Tu B, Zhao DY (2013) One-pot synthesis of thermally stable gold@mesoporous silica core-shell nanospheres with catalytic activity. Nano Res 6:871–879
Choi Y, Bae HS, Seo E, Jang S, Park KH, Kim BS (2011) Hybrid gold nanoparticle-reduced graphene oxide nanosheets as active catalysts for highly efficient reduction of nitroarenes. J Mater Chem 21:15431–15436
Fan CM, Zhang LF, Wang SS, Wang DH, Lu LQ, Xu AW (2012) Novel CeO2 yolk–shell structures loaded with tiny Au nanoparticles for superior catalytic reduction of p-nitrophenol. Nanoscale 4:6835–6840
Güttel R, Paul M, Schüth F (2010) Ex-post size control of high-temperature-stable yolk–shell Au,@ ZrO2 catalysts. Chem Commun 46:895–897
Han J, Wang MG, Chen R, Han N, Guo R (2014) Beyond yolk–shell nanostructure: a single Au nanoparticle encapsulated in the porous shell of polymer hollow spheres with remarkably improved catalytic efficiency and recyclability. Chem Commun 50:8295–8298
Hu XG, Dong SJ (2008) Metal nanomaterials and carbon nanotubes—synthesis, functionalization and potential applications towards electrochemistry. J Mater Chem 18:1279–1295
Lan GJ, Zhang XM, Zhang XM, Li MR, Li Y, Yang QH (2015) Yolk–shell nanospheres with soluble amino-polystyrene as a reservoir for Pd NPs. RSC Adv 5:35730–35736
Lee J, Park JC, Bang JU, Song H (2008a) Precise tuning of porosity and surface functionality in Au@SiO2 nanoreactors for high catalytic efficiency. Chem Mater 20:5839–5844
Lee J, Park JC, Song H (2008b) A nanoreactor framework of a Au@SiO2 Yolk/Shell structure for catalytic reduction of p-Nitrophenol. Adv Mater 20:1523–1528
Lee I, Joo JB, Yin YD, Zaera F (2011) A Yolk@Shell Nanoarchitecture for Au/TiO2 Catalysts. Angew Chem Int Ed 50:10208–10211
Lee SH, Rusakova I, Hoffman DM, Jacobson AJ, Lee TR (2013) Monodisperse SnO2-coated gold nanoparticles are markedly more stable than analogous SiO2-coated gold nanoparticles. ACS Appl Mater Interfaces 5:2479–2484
Li GD, Tang ZY (2014) Noble metal nanoparticle@metal oxide core/yolk–shell nanostructures as catalysts: recent progress and perspective. Nanoscale 6:3995–4011
Liu J, Qiao SZ, Hartono SB, Lu GQ (2010) Monodisperse Yolk-Shell Nanoparticles with a Hierarchical Porous Structure for Delivery Vehicles and Nanoreactors. Angew Chem Int Ed 49:4981–4985
Liu S, Chen GY, Prasad PN, Swihart MT (2011a) Synthesis of monodisperse Au, Ag, and Au–Ag alloy nanoparticles with tunable size and surface plasmon resonance frequency. Chem Mater 23:4098–4101
Liu J, Qiao SZ, Chen JS, Lou XW, Xing XR, Lu GQ (2011b) Yolk/shell nanoparticles: new platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem Commun 47:12578–12591
Liu R, Qu FL, Guo YL, Yao N, Priestley RD (2014) Au@carbon yolk–shell nanostructures via one-step core–shell–shell template. Chem Commun 50:478–480
Liu C, Li JS, Wang J et al (2015) Synthesis of Ag@SiO2 yolkshell nanoparticles for hydrogen peroxide detection. RSC Adv 5:17372–17378
Llevot A, Astruc D (2012) Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer. Chem Soc Rev 41:242–257
Niikura K, Iyo N, Matsuo Y, Mitomo H, Ijiro K (2013) Sub-100 nm gold nanoparticle vesicles as a drug delivery carrier enabling rapid drug release upon light irradiation. ACS Appl Mater Interfaces 5:3900–3907
Qi J, Chen J, Li GD, Li SX, Gao Y, Tang ZY (2012) Facile synthesis of core–shell Au@CeO2 nanocomposites with remarkably enhanced catalytic activity for CO oxidation. Energy Environ Sci 5:8937–8941
Rai P, Yoon JW, Jeong HM, Hwang SJ, Kwak CH, Lee JH (2014) Design of highly sensitive and selective Au@NiO yolk–shell nanoreactors for gas sensor applications. Nanoscale 6:8292–8299
Saha S, Pal A, Kundu S, Basu S, Pal T (2010) Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 26:2885–2893
Saha K, Agasti SS, Kim C, Li XN, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Sau TK, Rogach AL, Jäckel F, Klar TA, Feldmann J (2010) Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv Mater 22:1805–1825
Tang SC, Vongehr S, Meng XK (2010) Carbon spheres with controllable silver nanoparticle doping. J Phy Chem C 114:977–982
Tripathy SK, Jo JN, Kwang-Joong O, Han SD, Lee IH, Yu YT (2010) Optical studies of Au@SnO2/poly-(vinyl) alcohol core-shell nanoparticle thin films. J Mater Sci: Mater Electron 21:758–764
Tripathy SK, Jo JN, Song HM, Yu YT (2011) Fabrication and optical study of Ag@SnO2 core-shell structure nanoparticle thin films. Appl Phys A 104:601–607
Tripathy SK, Mishra A, Jha SK, Wahab R, Al-Khedhairy AA (2013) Synthesis of thermally stable monodispersed Au@SnO2 core–shell structure nanoparticles by a sonochemical technique for detection and degradation of acetaldehyde. Anal Methods 5:1456–1462
Wang DW, Tejerina B, Lagzi I, Kowalczyk B, Grzybowski BA (2010a) Bridging interactions and selective nanoparticle aggregation mediated by monovalent cations. ACS Nano 5:530–536
Wang HJ, Sun FQ, Zhang Y et al (2010b) Photochemical growth of nanoporous SnO2 at the air–water interface and its high photocatalytic activity. J Mater Chem 20:5641–5645
Wang SN, Zhang MC, Zhang WQ (2011) Yolk—Shell catalyst of single Au nanoparticle encapsulated within hollow mesoporous silica microspheres. ACS Catal 1:207–211
Wang XZ, Qiu S, He CZ, Lu GX, Liu W, Liu JR (2013) Synthesis of Au decorated SnO2 mesoporous spheres with enhanced gas sensing performance. RSC Adv 3:19002–19008
Xiong R, Wang YR, Zhang XX, Lu CH, Lan LD (2014) In situ growth of gold nanoparticles on magnetic γ-Fe2O3@cellulose nanocomposites: a highly active and recyclable catalyst for reduction of 4-nitrophenol. RSC Adv 4:6454–6462
Yao TJ, Cui TY, Fang X, Cui F, Wu J (2013) Preparation of yolk–shell FexOy/Pd@mesoporous SiO2 composites with high stability and their application in catalytic reduction of 4-nitrophenol. Nanoscale 5:5896–5904
Zhang XQ, Ren H, Wang TT et al (2012) Controlled synthesis and magnetically separable photocatalytic properties of magnetic iron oxides@SnO2 yolk-shell nanocapsules. J Mater Chem 22:13380–13385
Zheng JM, Dong YL, Wang WF et al (2013) In situ loading of gold nanoparticles on Fe3O4@SiO2 magnetic nanocomposites and their high catalytic activity. Nanoscale 5:4894–4901
Zhu CZ, Han L, Hu P, Dong SJ (2012) In situ loading of well-dispersed gold nanoparticles on two-dimensional graphene oxide/SiO2 composite nanosheets and their catalytic properties. Nanoscale 4:1641–1646
Acknowledgments
We would like to thank the National Natural Science Foundation of China (Grant Nos. 21173038, 21301027 and 21401013), Natural Science Foundation and Science and Technology Development Planning of Jilin Province (201215003, 201201115, 20130522136JH and 20140520088JH), Program for New Century Excellent Talents in University (NCET-13-0720), and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20132216120002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Li, L., Wang, C. et al. Facile approach to synthesize uniform Au@mesoporous SnO2 yolk–shell nanoparticles and their excellent catalytic activity in 4-nitrophenol reduction. J Nanopart Res 18, 2 (2016). https://doi.org/10.1007/s11051-015-3307-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3307-8